��Ŀ����
A��B��C��D��E�ֱ��dz������ᡢ��Σ���֪A�Ǻ���Ԫ�صĻ����E����ɫ����������֮�������¹�ϵ��
��2A+B�TC+2H2O ��C+D�TBaSO4��+2A ��D+CuSO4�TBaSO4��+E��
��1������������Ϣ���ƶ�д��B��E�Ļ�ѧʽ��B
��2��д��D��CuSO4��ѧ����ʽ
��2A+B�TC+2H2O ��C+D�TBaSO4��+2A ��D+CuSO4�TBaSO4��+E��
��1������������Ϣ���ƶ�д��B��E�Ļ�ѧʽ��B
H2SO4
H2SO4
��ECu��OH��2
Cu��OH��2
����2��д��D��CuSO4��ѧ����ʽ
Ba��OH��2+CuSO4�TBaSO4��+Cu��OH��2��
Ba��OH��2+CuSO4�TBaSO4��+Cu��OH��2��
��������������������ƶ��⣬Ҫ��������ṩ����Ϣ��ץס�����ͻ�ƿڽ��У���E����ɫ��������˵��E��������ͭ������Ʋ�ABCD�����ʣ�
����⣺��1��E����ɫ������˵��E��������ͭ������D+CuSO4�TBaSO4��+E�������ø��ֽⷴӦ���ص㣬˵��D��Ba��OH��2���ָ��ݣ�2��C+D�TBaSO4��+2A��A�Ǻ���Ԫ�صĻ����˵��A���������ƣ�C�������ƣ��ٸ��ݣ�1��2A+B�TC+2H2O��˵��B�����
��2�����ø��ֽⷴӦ���ص㡰�����ϣ������ϡ����ʿ���д��ѧ����ʽBa��OH��2+CuSO4�TBaSO4��+Cu��OH��2����
�ʴ�Ϊ����1��H2SO4��Cu��OH��2����2��Ba��OH��2+CuSO4�TBaSO4��+Cu��OH��2��
��2�����ø��ֽⷴӦ���ص㡰�����ϣ������ϡ����ʿ���д��ѧ����ʽBa��OH��2+CuSO4�TBaSO4��+Cu��OH��2����
�ʴ�Ϊ����1��H2SO4��Cu��OH��2����2��Ba��OH��2+CuSO4�TBaSO4��+Cu��OH��2��
�������ڽ��д��������ƶ���ʱ��Ҫ���������غ㶨�ɣ���ʹ��Ӧ�����С���

��ϰ��ϵ�д�
�����Ŀ
 A��B��C��D��E�ֱ���һ����̼��������̼��������������ϡ���������������Һ�е�һ�����ʣ������Բ��ʾ�������ʣ�����Բ���б�ʾ�������ʿ��Է�����ѧ��Ӧ����һ����ʾ�����ʼ��ת����ϵ�����ַ�Ӧ�������ͷ�Ӧ��������ȥ����������Dת��������E�Ĺ��������ȣ���ش��������⣺
A��B��C��D��E�ֱ���һ����̼��������̼��������������ϡ���������������Һ�е�һ�����ʣ������Բ��ʾ�������ʣ�����Բ���б�ʾ�������ʿ��Է�����ѧ��Ӧ����һ����ʾ�����ʼ��ת����ϵ�����ַ�Ӧ�������ͷ�Ӧ��������ȥ����������Dת��������E�Ĺ��������ȣ���ش��������⣺
 A��B��C��D��E�ֱ��Ƕ�����̼��ľ̿������ͭ��ϡ���ᡢ����������Һ�е�һ�����ʣ�����Բ�ཻ��ʾ�������ʿ��Է�����Ӧ���ش��������⣮
A��B��C��D��E�ֱ��Ƕ�����̼��ľ̿������ͭ��ϡ���ᡢ����������Һ�е�һ�����ʣ�����Բ�ཻ��ʾ�������ʿ��Է�����Ӧ���ش��������⣮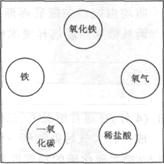 ��2009?��������A��B��C��D��E�ֱ���������������������һ����̼��ϡ�����е�һ�����ʣ���ش��������⣺
��2009?��������A��B��C��D��E�ֱ���������������������һ����̼��ϡ�����е�һ�����ʣ���ش��������⣺ A��B��C��D��E�ֱ��dz��л�ѧ��ͬ���ij������ʣ�����C��ˮ��Һ����ɫ��E������ʹȫ�������ů������֮���ϵ��ͼ��ʾ����-����ʾ�����������ܷ�����Ӧ����������ʾһ�����ʿ�ת��Ϊ��һ�����ʣ�����ش��������⡰
A��B��C��D��E�ֱ��dz��л�ѧ��ͬ���ij������ʣ�����C��ˮ��Һ����ɫ��E������ʹȫ�������ů������֮���ϵ��ͼ��ʾ����-����ʾ�����������ܷ�����Ӧ����������ʾһ�����ʿ�ת��Ϊ��һ�����ʣ�����ش��������⡰