��Ŀ����
�Ȼ��ƺ�̼���ƾ�����ܽ�ȣ�0�桫30�棩���±���ʾ��
| 0�� | 10�� | 20�� | 30�� | |
| NaCl��g�� | 35.7 | 35.8 | 36.0 | 36.3 |
| Na2CO3���壨g�� | 7.0 | 12.5 | 21.5 | 38.8 |
��2������ˮ��100g���Ȼ�����̼���Ƶı�����Һ����30�潵��0��ʱ�������Ȼ��ƾ��������______������ڡ���С�ڡ����ڡ���̼���ƾ����������
��3�����Ӻ�������NaCl���ʵ�̼����Ũ��Һ�з����̼���ƾ��壬Ӧ��ȡ______������
��4��20��ʱ��NaCl�ܽ���ˮ��ʵ���������±���������������ȷ����______��
| ʵ����� | ˮ��������g�� | ����NaCl��������g�� | ��Һ��������g�� |
| �� | 10 | 2 | 12 |
| �� | 10 | 3 | 13 |
| �� | 10 | 4 | 13.6 |
| �� | 10 | 5 | 13.6 |
C��20��ʱ10gˮ������ܽ�4g NaCl������D���ۢ���Һ����������������ȣ�
�⣺��1���б�����Կ���̼���Ƶ��ܽ�����¶����߶����ʴ�Ϊ�����߶�����
��2��̼���Ƶ��ܽ�����¶ȱ仯�����ԣ����Ȼ������¶ȱ仯�����ԣ�������30�潵��0��ʱ�Ȼ��������ľ�����٣��ʴ�Ϊ��С��
��3��̼���Ƶ��ܽ�����¶ȱ仯�����ԣ����Ȼ������¶ȱ仯�����ԣ��ɲ��ýᾧ�����ķ�ʽ���У��ʴ�Ϊ����ȴ�ȱ�����Һ������������
��4��A����20��ʱ�Ȼ��Ƶ��ܽ����36g��֪10gˮ������ܽ�3.6gʳ�Σ������������������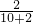 ��100%=16.7%���ʴ�ѡ�����
��100%=16.7%���ʴ�ѡ�����
B��ͬ��3g�Ȼ��ƻ�ȫ���ܽ�10gˮ�У��γɲ�������Һ���ʴ�ѡ����ȷ��
C�������Ϸ���֪10gˮ������ܽ�3.6gʳ�Σ��ʴ�ѡ�����
D���ܾۢ��γ�����һ�¶��µı�����Һ������������������ͬ���ʴ�ѡ����ȷ��
��ѡB��D
��������1������ͼ���ṩ���¶����ܽ�ȵ�������ϵ������⣮
��2��ͨ��0����30��ʱ�������ʵ��ܽ����ֵ�����жϾ������������Ĺ�ϵ��
��3�������Ȼ�����̼���Ƶ��ܽ�����¶ȱ仯�����ƣ�������ȴ�ȱ�����Һ�ķ�ʽ�ɽ��У�
��4����ϸ������ʵ��ܽ����ֵ���ص��ж������Ƿ���ȫ���ܽ����ȷ����
�����������ǽ������ʵ��ܽ�ȱ�����е��������Ŀ��飬ֻҪ�Ա����ṩ�����ݽ�����ϸ�ķ���̽�֣���Ŀ�Ϳɽ����
��2��̼���Ƶ��ܽ�����¶ȱ仯�����ԣ����Ȼ������¶ȱ仯�����ԣ�������30�潵��0��ʱ�Ȼ��������ľ�����٣��ʴ�Ϊ��С��
��3��̼���Ƶ��ܽ�����¶ȱ仯�����ԣ����Ȼ������¶ȱ仯�����ԣ��ɲ��ýᾧ�����ķ�ʽ���У��ʴ�Ϊ����ȴ�ȱ�����Һ������������
��4��A����20��ʱ�Ȼ��Ƶ��ܽ����36g��֪10gˮ������ܽ�3.6gʳ�Σ������������������
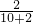 ��100%=16.7%���ʴ�ѡ�����
��100%=16.7%���ʴ�ѡ�����B��ͬ��3g�Ȼ��ƻ�ȫ���ܽ�10gˮ�У��γɲ�������Һ���ʴ�ѡ����ȷ��
C�������Ϸ���֪10gˮ������ܽ�3.6gʳ�Σ��ʴ�ѡ�����
D���ܾۢ��γ�����һ�¶��µı�����Һ������������������ͬ���ʴ�ѡ����ȷ��
��ѡB��D
��������1������ͼ���ṩ���¶����ܽ�ȵ�������ϵ������⣮
��2��ͨ��0����30��ʱ�������ʵ��ܽ����ֵ�����жϾ������������Ĺ�ϵ��
��3�������Ȼ�����̼���Ƶ��ܽ�����¶ȱ仯�����ƣ�������ȴ�ȱ�����Һ�ķ�ʽ�ɽ��У�
��4����ϸ������ʵ��ܽ����ֵ���ص��ж������Ƿ���ȫ���ܽ����ȷ����
�����������ǽ������ʵ��ܽ�ȱ�����е��������Ŀ��飬ֻҪ�Ա����ṩ�����ݽ�����ϸ�ķ���̽�֣���Ŀ�Ϳɽ����

��ϰ��ϵ�д�
�����Ŀ
9���Ȼ��ƺ�̼���ƾ�����ܽ�ȣ�0�桫30�棩���±���ʾ��
��̼���ƾ�����ܽ�����¶�Ӱ��ϴ� �ڽ���ˮ��1OOg���Ȼ�����̼���Ƶı�����Һ����30�潵��O�棬���������������ȣ� ���Ȼ��ƺ�̼���ƾ�����ܽ�����ʱ����Ӧ���¶���2O�桫30��֮�䣻 �����Ӻ�������NaCl���ʵ�̼����Ũ��Һ�з����̼���ƾ��壬Ӧ��ȡ������Һ�¶ȵķ�ʽ�� ����˵���У���ȫ��ȷ��һ���ǣ������� |
�Ȼ��ƺ�̼���ƾ�����ܽ�ȣ�0�桫30�棩���±���ʾ��
��1�������ϱ�������̼���ƾ�����ܽ�����¶� ��
��2������ˮ��100g���Ȼ�����̼���Ƶı�����Һ����30�潵��0��ʱ�������Ȼ��ƾ�������� ������ڡ���С�ڡ����ڡ���̼���ƾ����������
��3�����Ӻ�������NaCl���ʵ�̼����Ũ��Һ�з����̼���ƾ��壬Ӧ��ȡ ������
��4��20��ʱ��NaCl�ܽ���ˮ��ʵ���������±���������������ȷ���� ��
A����������Һ��������������Ϊ20% B������������Һ�Dz�������Һ
C��20��ʱ10gˮ������ܽ�4g NaCl D���ۢ���Һ����������������ȣ�
| 0�� | 10�� | 20�� | 30�� | |
| NaCl��g�� | 35.7 | 35.8 | 36.0 | 36.3 |
| Na2CO3���壨g�� | 7.0 | 12.5 | 21.5 | 38.8 |
��2������ˮ��100g���Ȼ�����̼���Ƶı�����Һ����30�潵��0��ʱ�������Ȼ��ƾ��������
��3�����Ӻ�������NaCl���ʵ�̼����Ũ��Һ�з����̼���ƾ��壬Ӧ��ȡ
��4��20��ʱ��NaCl�ܽ���ˮ��ʵ���������±���������������ȷ����
| ʵ����� | ˮ��������g�� | ����NaCl��������g�� | ��Һ��������g�� |
| �� | 10 | 2 | 12 |
| �� | 10 | 3 | 13 |
| �� | 10 | 4 | 13.6 |
| �� | 10 | 5 | 13.6 |
C��20��ʱ10gˮ������ܽ�4g NaCl D���ۢ���Һ����������������ȣ�


