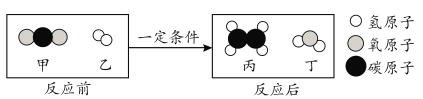
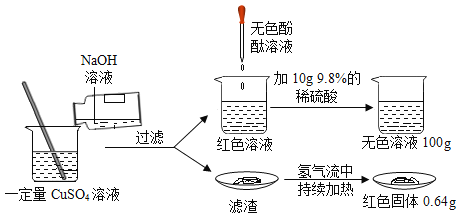
��Ŀ����
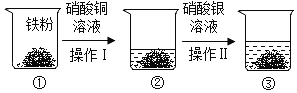
��ѧʵ��������һƿ��ɫҺ�� A ��һ���������� B��С�ͬѧ��̽�����Ǹ��Եijɷ���ɡ���֪��Ʒ A �п��ܺ��� KNO3��HCl��BaCl2��Ba(NO3)2 �е�һ�ֻ���֣���Ʒ B �п��� ���� CaO �� CaCl2 ��һ�ֻ����֡�С�ͬѧ����ͼ��ʾ������ʵ��̽�������ֵ�������ͼ��������������������п��ܷ����ķ�Ӧ��ǡ����ȫ���У�
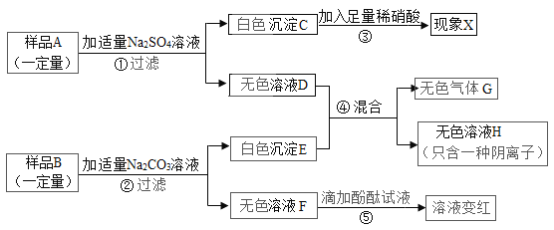
����������Ϣ���Իش��������⣺
��1������ C �Ļ�ѧʽΪ___________������ X Ϊ___________�� ��Һ F �� pH___________7��������� ��������������
��2����Һ D �У�һ���������ڵ���������___________ (д���ӷ���)��
��3��д��������з�����Ӧ��һ����ѧ����ʽ��___________��
��4������������Ϣ������֪��Ʒ B ��һ�����ڵ�������___________ (д��ѧʽ)��
��5������������Ϣ������֪��Ʒ A ��һ�����ڵ�������________ (д��ѧʽ)�� ���������_______
��ϰ��ϵ�д�
 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ