��Ŀ����
 2010���Ϻ�������ij��ݽ���ʹ���˴����Ľ������ϣ�
2010���Ϻ�������ij��ݽ���ʹ���˴����Ľ������ϣ�
��1����д��һ����ҵ�������Ļ�ѧ����ʽ______��
��2��������ij��ݽ���ʹ���˴����ĸֲĶ����Ǵ�������Ҫ����Ϊ�ֺʹ���������______���������ܣ�������Щ��ͬ���ܵ�ԭ����______��
��3����ͼ���Ϻ������÷Ͼɽ��������ij��е��ܣ���д�����ַ�ֹ��Щ���ܸ�ʴ�ķ���______��
��4������ͬѧ�ռ��������������᳡�ݵĽ�����Ƭ���ֱ���ΪX��Y��Z��������X��Y�ֱ����ϡ�����У�Y��Һ����������X����Ӧ����X��Z�ֱ������������Һ�У���һ�������X����������������Zû�б仯����������ʵ����ʵ������ΪX��Y��Z�Ľ������˳��Ϊ______����д��һ�����������X����������Ӧ�Ļ�ѧ����ʽΪ______����XҪ�þ������ʱ�ʾ����
��5���Ϻ���ij�������÷������ᷴӦ������������������1t����м�����ʲ��μӷ�Ӧ����ͬ������9.8t������ǡ�÷�Ӧ�����õ�0.78t����ʣ�����ù������÷��������������Ϊ���٣�
�⣺��1����ҵ������ԭ������������һ����̼��Ӧ�������������̼���䷽��ʽΪ��Fe2O3+3CO 2Fe+3CO2��
2Fe+3CO2��
��2�����ڸ��к�̼�����ĺϽ����Ըֵ�Ӳ�ȱ���Ҫ��
��3����������ĸ�ʴһ�����������꣬���Կ��Բ��ø�����Ϳ������ڽ�����������һ�������Ӧ�Ľ�������Ĥ�Է�ֹ��������ʴ��
��4�����ݽ������˳�������ã�������֮ǰ�Ľ�������ϡ�����ϡ���ᷴӦ����������X��Y�ֱ����ϡ�����У�Y��Һ����������X����Ӧ�����Խ������y��x������ǰ��Ľ��������ء��ơ����⣩�ɽ����ں���Ľ�����������Һ���û���������X��Z�ֱ������������Һ�У���һ�������X����������������Zû�б仯�����Խ������y��x���ۺϿ�֪���ߵĽ������˳����y��x��z������ͭ�Ļ������������Ա���ǿ������X����������Ӧ�Ļ�ѧ����ʽΪ��Cu+2AgNO3�TCu��NO3��2+2Ag��
��5��������֪ʣ������Dz��������ᷴӦ�����ʣ����Բ��뷴Ӧ������������1t-0.78t=0.22t
����뷴Ӧ�������������x
Fe+H2SO4=FeSO4+H2��
56 98
0.22t x
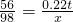
x=0.385t
��ù������÷��������������Ϊ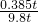 ��100%��3.9%
��100%��3.9%
�ʴ�Ϊ����1��Fe2O3+3CO 2Fe+3CO2��
2Fe+3CO2��
��2��Ӳ�ȴ��Ժã��������ĺϽ�
��3��Ϳ�����ƣ�
��4��y��x��z��Cu+2AgNO3�TCu��NO3��2+2Ag��
��5������ù������÷��������������Ϊ3.9%��
��������1�����ݹ�ҵ������ԭ������������һ����̼��Ӧ��д����ʽ��
��2�����ݸֵijɷּ��������ʵ��ص�������
��3�����ݸ�ʴ������ԭ���жϷ�ֹ����ʴ�ķ�����
��4�����ݽ������˳�������ã�������֮ǰ�Ľ�������ϡ�����ϡ���ᷴӦ��������������ǰ��Ľ��������ء��ơ����⣩�ɽ����ں���Ľ�����������Һ���û�������
��5�����ݻ�ѧ����ʽ�Ļ�����������Һ���������������ļ��㷽�����㼴�ɣ�
�����������Ƕ��������ص�֪ʶ�Ŀ����⣬����Ĺؼ��Ƕ���ػ���֪ʶ�����������գ������˻���֪ʶ����Ҫ�ԣ�
 2Fe+3CO2��
2Fe+3CO2����2�����ڸ��к�̼�����ĺϽ����Ըֵ�Ӳ�ȱ���Ҫ��
��3����������ĸ�ʴһ�����������꣬���Կ��Բ��ø�����Ϳ������ڽ�����������һ�������Ӧ�Ľ�������Ĥ�Է�ֹ��������ʴ��
��4�����ݽ������˳�������ã�������֮ǰ�Ľ�������ϡ�����ϡ���ᷴӦ����������X��Y�ֱ����ϡ�����У�Y��Һ����������X����Ӧ�����Խ������y��x������ǰ��Ľ��������ء��ơ����⣩�ɽ����ں���Ľ�����������Һ���û���������X��Z�ֱ������������Һ�У���һ�������X����������������Zû�б仯�����Խ������y��x���ۺϿ�֪���ߵĽ������˳����y��x��z������ͭ�Ļ������������Ա���ǿ������X����������Ӧ�Ļ�ѧ����ʽΪ��Cu+2AgNO3�TCu��NO3��2+2Ag��
��5��������֪ʣ������Dz��������ᷴӦ�����ʣ����Բ��뷴Ӧ������������1t-0.78t=0.22t
����뷴Ӧ�������������x
Fe+H2SO4=FeSO4+H2��
56 98
0.22t x
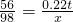
x=0.385t
��ù������÷��������������Ϊ
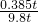 ��100%��3.9%
��100%��3.9%�ʴ�Ϊ����1��Fe2O3+3CO
 2Fe+3CO2��
2Fe+3CO2����2��Ӳ�ȴ��Ժã��������ĺϽ�
��3��Ϳ�����ƣ�
��4��y��x��z��Cu+2AgNO3�TCu��NO3��2+2Ag��
��5������ù������÷��������������Ϊ3.9%��
��������1�����ݹ�ҵ������ԭ������������һ����̼��Ӧ��д����ʽ��
��2�����ݸֵijɷּ��������ʵ��ص�������
��3�����ݸ�ʴ������ԭ���жϷ�ֹ����ʴ�ķ�����
��4�����ݽ������˳�������ã�������֮ǰ�Ľ�������ϡ�����ϡ���ᷴӦ��������������ǰ��Ľ��������ء��ơ����⣩�ɽ����ں���Ľ�����������Һ���û�������
��5�����ݻ�ѧ����ʽ�Ļ�����������Һ���������������ļ��㷽�����㼴�ɣ�
�����������Ƕ��������ص�֪ʶ�Ŀ����⣬����Ĺؼ��Ƕ���ػ���֪ʶ�����������գ������˻���֪ʶ����Ҫ�ԣ�

��ϰ��ϵ�д�
�����Ŀ
 2��2010���Ϻ��������й���--������֮�ڡ�����ͼ��������ṹ���ù���Q460���Ƴɵģ������й�Q460�ֵ������У����ڻ�ѧ���ʵ��ǣ�������
2��2010���Ϻ��������й���--������֮�ڡ�����ͼ��������ṹ���ù���Q460���Ƴɵģ������й�Q460�ֵ������У����ڻ�ѧ���ʵ��ǣ�������