题目内容
(2007?花都区一模)只用Ca、C、O、Cl、H五种元素的一种或几种,按要求填空:
(1)各写出一个化学式:
①氧化物
(2)用上述元素组成的物质,按要求各写出一个化学方程式:
①分解反应
(3)实验室购进一药品,该药品主要成份是由上述元素中三种元素组成的一种难溶盐.老师将药品交给学生测定其中该盐的质量分数.同学们取样品,并加入10%的稀盐酸,直到不再产生气泡为止,共用去稀盐酸60g.请同学计算参加反应的该盐的质量
(1)各写出一个化学式:
①氧化物
CaO
CaO
②酸HCl
HCl
③碱Ca(OH)2
Ca(OH)2
④盐CaCl2
CaCl2
(2)用上述元素组成的物质,按要求各写出一个化学方程式:
①分解反应
H2CO3═H2O+CO2↑
H2CO3═H2O+CO2↑
②复分解反应CaCO3+2HCl=CaCl2+H2O+CO2↑
CaCO3+2HCl=CaCl2+H2O+CO2↑
(3)实验室购进一药品,该药品主要成份是由上述元素中三种元素组成的一种难溶盐.老师将药品交给学生测定其中该盐的质量分数.同学们取样品,并加入10%的稀盐酸,直到不再产生气泡为止,共用去稀盐酸60g.请同学计算参加反应的该盐的质量
0.8g
0.8g
(计算结果精确到0.1).同学们最后还无法得出药品中该盐的质量分数,其原因是不知道药品的质量
不知道药品的质量
.分析:(1)①氧化物:由两种元素组成且其中一种是氧元素的化合物,如二氧化碳,一氧化碳,氧化钙等;
②酸:电离时产生的阳离子全都是氢离子的化合物,如盐酸、硫酸、碳酸等;
③碱:电离时产生的阴离子全都是氢氧根离子的化合物,如氢氧化钙、氢氧化钠等;
④盐:电离时生成金属阳离子(或NH4)和酸根离子的化合物,如碳酸钠、氯化钙,硝酸铵等;
(2)①分解反应:一种化合物在特定条件下分解成二种或二种以上物质的反应;可简记为AB=A+B;如电解水,碳酸分解;
②复分解反应:由两种化合物互相交换成分,生成另外两种化合物的反应;可简记为AB+CD=AD+CB;如氢氧化钙溶液与盐酸反应,碳酸钙与盐酸反应;
(3)碳酸钙是一种不溶于水的盐,再根据碳酸钙与盐酸反应的方程式进行计算,无法得出药品中该盐的质量分数,其原因是不知道样品的质量.
②酸:电离时产生的阳离子全都是氢离子的化合物,如盐酸、硫酸、碳酸等;
③碱:电离时产生的阴离子全都是氢氧根离子的化合物,如氢氧化钙、氢氧化钠等;
④盐:电离时生成金属阳离子(或NH4)和酸根离子的化合物,如碳酸钠、氯化钙,硝酸铵等;
(2)①分解反应:一种化合物在特定条件下分解成二种或二种以上物质的反应;可简记为AB=A+B;如电解水,碳酸分解;
②复分解反应:由两种化合物互相交换成分,生成另外两种化合物的反应;可简记为AB+CD=AD+CB;如氢氧化钙溶液与盐酸反应,碳酸钙与盐酸反应;
(3)碳酸钙是一种不溶于水的盐,再根据碳酸钙与盐酸反应的方程式进行计算,无法得出药品中该盐的质量分数,其原因是不知道样品的质量.
解答:解:
(1)①氧化物:由两种元素组成且其中一种是氧元素的化合物,如CaO;
②酸:电离时产生的阳离子全都是氢离子的化合物,如HCl;
③碱:电离时产生的阴离子全都是氢氧根离子的化合物,如Ca(OH)2;
④盐:电离时生成金属阳离子(或NH4)和酸根离子的化合物,如CaCl2;
(2)①分解反应:符合AB=A+B,如碳酸分解的化学方程式为H2CO3═H2O+CO2↑;
②复分解反应:符合AB+CD=AD+CB,如碳酸钙与盐酸反应的方程式为CaCO3+2HCl=CaCl2+H2O+CO2↑;
(3)参加反应的盐酸溶质的质量是60g×10%=0.6g
设参加反应的碳酸钙质量是X
CaCO3+2HCl=CaCl2+H2O+CO2↑
100 73
X 0.6g
=
X=0.8g
药品中该盐的质量分数等于碳酸钙的质量除以药品的质量,不知道药品的质量,所以计算不出碳酸钙的质量分数.
故答案为:
(1)①CaO;②HCl;③Ca(OH)2;④CaCl2;
(2)①H2CO3═H2O+CO2↑;
②CaCO3+2HCl=CaCl2+H2O+CO2↑;
(3)0.8g;不知道药品的质量.
(1)①氧化物:由两种元素组成且其中一种是氧元素的化合物,如CaO;
②酸:电离时产生的阳离子全都是氢离子的化合物,如HCl;
③碱:电离时产生的阴离子全都是氢氧根离子的化合物,如Ca(OH)2;
④盐:电离时生成金属阳离子(或NH4)和酸根离子的化合物,如CaCl2;
(2)①分解反应:符合AB=A+B,如碳酸分解的化学方程式为H2CO3═H2O+CO2↑;
②复分解反应:符合AB+CD=AD+CB,如碳酸钙与盐酸反应的方程式为CaCO3+2HCl=CaCl2+H2O+CO2↑;
(3)参加反应的盐酸溶质的质量是60g×10%=0.6g
设参加反应的碳酸钙质量是X
CaCO3+2HCl=CaCl2+H2O+CO2↑
100 73
X 0.6g
| 100 |
| X |
| 73 |
| 0.6g |
X=0.8g
药品中该盐的质量分数等于碳酸钙的质量除以药品的质量,不知道药品的质量,所以计算不出碳酸钙的质量分数.
故答案为:
(1)①CaO;②HCl;③Ca(OH)2;④CaCl2;
(2)①H2CO3═H2O+CO2↑;
②CaCO3+2HCl=CaCl2+H2O+CO2↑;
(3)0.8g;不知道药品的质量.
点评:本题主要考查了化学方程式的书写和应用,及分解反应和复分解反应及其应用和对氧化物、酸、碱、盐的概念的理解、应用,要掌握相关知识才能正确解答.

练习册系列答案
相关题目

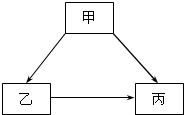 (2007?花都区一模)甲、乙、丙是初中化学中常见的物质,其转化关系如下图所示:
(2007?花都区一模)甲、乙、丙是初中化学中常见的物质,其转化关系如下图所示: