��Ŀ����
�Ϻ����ҹ��Ĺ����캣���Ϻ��̲��ŷḻ�ĺ�����Դ��
��1���ҹ���ѧ�����Ϻ���������һ���̲�����������Ϳ����Դ--��Ȼ��ˮ���������Ȼ������Ҫ�ɷ���CH4����ˮ�γɵı�״���壬����ȼ�գ��ֳơ���ȼ������������Ϊ��һ�������Դ��ԭ����
��2���Ӻ�ˮ����ȡ����þ�Ĺ�������ͼ��ʾ��

������ת�������У��������Ļ�ѧ��Ӧ���ڸ��ֽⷴӦ���� ���Ӧ˳���ţ���21���ͽ�����]
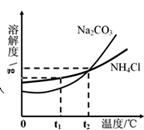
��3���ҹ���ѧ��ѧ�Һ�°����ĺ����Ƽ�������Ժ�ˮ��ɹ�Ρ��õ����Ȼ���Ϊԭ�ϣ�ͬʱ�Ƶ�Na2CO3��NH4Cl��Ʒ��Na2CO3��NH4Cl���ܽ��������ͼ��ʾ���ش��������⣺
��t1��ʱ��NH4Cl���ܽ�� Na2CO3���ܽ�ȣ�����ڡ���С�ڡ������ڡ�֮һ����
��t2��ʱ��Na2CO3������Һ�������������� NH4Cl������Һ��������������������ڡ���С�ڡ������ڡ�֮һ����
��t1��ʱ��һС�Թ���ʢ��Na2CO3�ı�����Һ���Թܵײ�����������Na2CO3���壬��С�Թܷ���ʢ��ˮ���ձ��У��ֽ�������NaOH��������ձ��ڵ�ˮ�У�С�Թ��ڵĹ����������ܽ⣬ԭ���� ![]()
��1��ȼ�ղ�����ˮ�Ͷ�����̼������Ⱦ����
��2���٢�
��3���ٴ��ڢڵ��ڢ� ������������ˮ�ų��������¶����ߣ�̼���Ƶ��ܽ������Ϊ��������Һ�����������ܽ�̼������



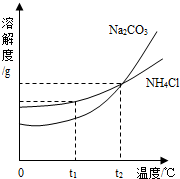 ��3���ҹ���ѧ��ѧ�Һ�°����ĺ����Ƽ�������Ժ�ˮ��ɹ�Ρ��õ����Ȼ���Ϊԭ�ϣ�ͬʱ�Ƶ�Na2CO3��NH4Cl��Ʒ��Na2CO3��NH4Cl���ܽ��������ͼ��ʾ���ش��������⣺
��3���ҹ���ѧ��ѧ�Һ�°����ĺ����Ƽ�������Ժ�ˮ��ɹ�Ρ��õ����Ȼ���Ϊԭ�ϣ�ͬʱ�Ƶ�Na2CO3��NH4Cl��Ʒ��Na2CO3��NH4Cl���ܽ��������ͼ��ʾ���ش��������⣺

