��Ŀ����
����Ŀ��������ѧ��֪ʶ��������������ش����⣺
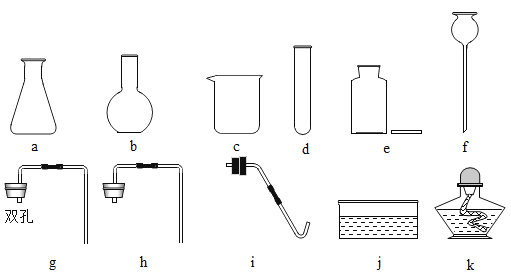
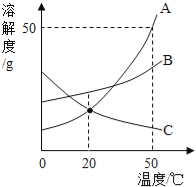
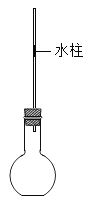
��1��д���������������ƣ�a_____ ��f_____��
��2��ʵ����ѡȡd��e��h��j����������ȡ���ռ�����ʱ����Ӧ�Ļ�ѧ����ʽΪ_____��
��3��С��ͬѧ��ʵ������ȡ������̼������ѡ��������b��g ���ѡ�����������е�_____ ����д��ţ��������װ�õ������Ժ�Ӧ��������b�м����ҩƷ��_____ ����Ӧ�Ļ�ѧ����ʽ��_____��
��4������һƿ������̼�Ƿ��Ѿ������ķ�����_____ ����֤�������Ƕ�����̼�ķ�����_____���û�ѧ����ʽ��д����
���𰸡���ƿ ����©�� 2H2O2![]() 2H2O+O2�� f��e ʯ��ʯ�������ʯ�� CaCO3+2HCl=CaCl2+H2O+CO2�� ����ȼ�ŵ�ľ������ƿ�ڣ����Ƿ�Ϩ�� CO2+Ca��OH��2=CaCO3��+H2O
2H2O+O2�� f��e ʯ��ʯ�������ʯ�� CaCO3+2HCl=CaCl2+H2O+CO2�� ����ȼ�ŵ�ľ������ƿ�ڣ����Ƿ�Ϩ�� CO2+Ca��OH��2=CaCO3��+H2O
��������
��1������a����������ƿ������b�������dz���©����
��2��ʵ����ѡȡd��e��h��f����������ȡ���ռ�����ʱ������Ҫ���ȣ���˫��ˮ�Ͷ�������������ʱ������Ҫ���ȣ����������ڶ�������������������������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2![]() 2H2O+O2����
2H2O+O2����
��3��ʵ������ȡCO2�����ڳ����£��ô���ʯ��ʯ��ʯ��ϡ������ȡ�ģ��÷�Ӧ�ķ�Ӧ���ǹ����Һ�壬����Ҫ���ȣ�С��ͬѧ��ʵ������ȡ������̼������ѡ��������b��g���ѡ�����������е�f��e������ҩƷһ���ȼ������ҩƷ���ټ���Һ��ҩƷ�������װ�õ������Ժ�Ӧ��������b�м����ҩƷ�Ǵ���ʯ��ʯ��ʯ������ʯ��ʯ��ʯ��Ҫ�ɷ���̼��ƣ�̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
��4��������̼����ȼ��Ҳ����֧��ȼ�գ���ʹȼ�ŵ�ľ��Ϩ�𡣶�����̼�����������ǣ���һ��ȼ�ŵ�ľ��ƽ���ڼ���ƿ�ڣ�ľ��Ϩ��֤�����ˣ�������̼һ���ó����ʯ��ˮ���飺������ͨ������ʯ��ˮ�У�ʯ��ˮ����ǣ�֤���Ƕ�����̼����Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��

 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�����Ŀ��������ij�о�С��̽��Ӱ�췴Ӧ���ʲ������ص����ʵ�����ݡ�
ʵ����� | H2O2��ҺŨ��% | H2O2��Һ���/mL | �¶�/�� | MmO2������/g | �ռ����������/mL | ��Ӧ�����ʱ��/s |
�� | 5 | 1 | 20 | 0.1 | 4 | 16.75 |
�� | 15 | 1 | 20 | 0.1 | 4 | 6.04 |
�� | 30 | 5 | 35 | 0 | 2 | 49.21 |
�� | 30 | 5 | 55 | 0 | 2 | 10.76 |
(1)ͨ��ʵ��ٺ͢ڶԱȿ�֪����ѧ��Ӧ������_____�йأ���ʵ��_____��_____�Աȿ�֪����ѧ��Ӧ�������¶ȵĹ�ϵ�ǣ�_____��
(2)��ѧ��Ӧ��ʵ������������Ӵ�����ײ�Ľ������ѧ��Ӧ�����������ӵĸ����йء��Դ��۽ǶȽ�������Ӧ��Ũ��Խ��ѧ��Ӧ����Խ������ԭ���ǣ�_____��
(3)��һ����15%�Ĺ���������Һ��������Ϊ�˼�С��Ӧ���ʣ��ɼ�������ˮϡ�ͣ�����������������_____(ѡ������С����������������������)��
(4)д��������Ӧ���ֱ���ʽ_____��