��Ŀ����
ͨ�����л�ѧѧϰ������������һЩ��ѧ֪ʶ������������ѧ֪ʶ��������������е�һЩ���⣮
��1����װ�ķ����ڿ������������н϶�ļ�ȩ��CH2O�����ж����ʣ���ȩ����______���������л�������ڰ�����װ����֮ǰ������ȡͨ��ȴ�ʩ�⣬�������á��ⴥý�����������ڹ��TiO2�������������£�����ȩ������е�ij���ʷ�Ӧ�������Ķ�����̼��ˮ���䷴Ӧ�Ļ�ѧ����ʽ��______��
��2���ճ������п���______��ȥ��ˮƿ�е�ˮ����
��3��ClO2��Ϊһ����������ˮ������������Cl2��NaClO2��ȡClO2��ͬʱ����NaCl���÷�Ӧ�Ļ�ѧ����ʽ��______��
��4���ӿα�������֪������������ʱ�������ģ�����Խ�����Դ�Ŀ�����������һ���������Ľ���______��
�⣺��1����ȩ�к���̼Ԫ�أ������л������л��
��ȩ�ܺͿ����е�������Ӧ����ˮ�Ͷ�����̼����ѧ����ʽΪ��CH2O+O2 CO2+H2O��
CO2+H2O��
��2���ճ������п���ʳ�׳�ȥ��ˮƿ�е�ˮ�������ʳ�ף�
��3��Cl2��NaClO2��Ӧ������ClO2��NaCl����Ӧ�Ļ�ѧ����ʽΪ��Cl2+2NaClO2=2ClO2+2NaCl��
��4���мƻ��������ؿ��ɿ���Ҳ�ǶԽ�����Դ��һ�ֱ���������мƻ��������ؿ��ɿ��
��������1������̼Ԫ�صĻ����������л����ȩ�ܺͿ����е�������Ӧ����ˮ�Ͷ�����̼��
��2��ʳ�������ԣ��ܺ�ˮ���е�̼��ơ�������þ��Ӧ��
��3��Cl2��NaClO2��Ӧ������ClO2��NaCl��
��4��������Դ���ڲ���������Դ��
������������Ҫ�������ʵ����ʺ���;��ȩ��ѧ����ʽ����д�ȷ����֪ʶ����д��ѧ����ʽʱҪע����ѭ�����غ㶨�ɣ�
��ȩ�ܺͿ����е�������Ӧ����ˮ�Ͷ�����̼����ѧ����ʽΪ��CH2O+O2
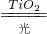 CO2+H2O��
CO2+H2O�� ��2���ճ������п���ʳ�׳�ȥ��ˮƿ�е�ˮ�������ʳ�ף�
��3��Cl2��NaClO2��Ӧ������ClO2��NaCl����Ӧ�Ļ�ѧ����ʽΪ��Cl2+2NaClO2=2ClO2+2NaCl��
��4���мƻ��������ؿ��ɿ���Ҳ�ǶԽ�����Դ��һ�ֱ���������мƻ��������ؿ��ɿ��
��������1������̼Ԫ�صĻ����������л����ȩ�ܺͿ����е�������Ӧ����ˮ�Ͷ�����̼��
��2��ʳ�������ԣ��ܺ�ˮ���е�̼��ơ�������þ��Ӧ��
��3��Cl2��NaClO2��Ӧ������ClO2��NaCl��
��4��������Դ���ڲ���������Դ��
������������Ҫ�������ʵ����ʺ���;��ȩ��ѧ����ʽ����д�ȷ����֪ʶ����д��ѧ����ʽʱҪע����ѭ�����غ㶨�ɣ�

��ϰ��ϵ�д�
 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
�����Ŀ