��Ŀ����
�����������γ�������������֮һ����ѧС���ͬѧ��Զ�����������о������ݻ�ѧ����ʽZn+2H2SO4 ŨZnSO4+SO2��+2H2O�����ȡһ������п����98%��Ũ����ǡ����ȫ��Ӧ��
ŨZnSO4+SO2��+2H2O�����ȡһ������п����98%��Ũ����ǡ����ȫ��Ӧ��������⣺�����Ƶõ����壬��ͬѧ��Ϊ���ܺ������ʣ�
��������裺��ѧС���Ƶõ�SO2�л��е�������Ҫ��H2��������һ�������Ҫԭ����______���û�ѧ����ʽ�ͱ�Ҫ������˵������
�������ϣ���1��CO2����ˮ��______��Ӧ��SO2��CO2һ����Ҳ�ܣ�
��2��H2��COһ�����ܽ�����������ұ���ɽ�����
��3��CaSO3������ˮ
ʵ����֤��Ϊ֤ʵ��ط�������ѧС���ͬѧ�������ͼ��ʾװ�ý���ʵ�飮

��1��Bװ�õ�������______��
��2����������к�����������Eװ���й۲쵽��������______����Ӧ�Ļ�ѧ����ʽΪ______ Cu+H2O
���𰸡�������������Ŀ����Ϣ��֪��п��Ũ���ᷴӦ���IJ������ᣬ������ˮ��ʹ��ҺŨ�Ƚ��ͳ�ϡ���������̼�Ļ�ѧ�����У�CO2����ˮ���������Һ��Ӧ�ȣ�Bװ�õ������dz�ȥ���������������л�ԭ�ԣ���������к�����������Eװ���й۲쵽�������ǣ�����ͭ�ɺ�ɫ��Ϊ��ɫ���������ڱ���ˮ��������������ͭ��Ӧ����ͭ��ˮ����������װ�ÿ��Բⶨ��������ж�������������������ȣ�Ҫ�ﵽ��һĿ�ģ�ʵ��С�����ⶨ����ֵ�ǣ��μӷ�Ӧ��п��������װ��B������������
����⣺��������裺п��Ũ���ᷴӦ���IJ������ᣬ������ˮ��ʹ��ҺŨ�Ƚ��ͳ�ϡ����ʴ�Ϊ��п��Ũ���ᷴӦ���IJ������ᣬ������ˮ��ʹ��ҺŨ�Ƚ��ͳ�ϡ���ᣬ����Zn+H2SO4��ϡ��=ZnSO4+H2��
�������ϣ�������̼�Ļ�ѧ�����У�CO2����ˮ���������Һ��Ӧ�ȣ��ʴ�Ϊ������Һ��д�������ƻ��������ƣ�
ʵ����֤����1�������������ɿ�����Ⱦ��Bװ�õ������dz�ȥ�������ʴ�Ϊ����ȥ��������
��2���������л�ԭ�ԣ���������к�����������Eװ���й۲쵽�������ǣ�����ͭ�ɺ�ɫ��Ϊ��ɫ���������ڱ���ˮ��������������ͭ��Ӧ����ͭ��ˮ���ʴ�Ϊ������ͭ�ɺ�ɫ��Ϊ��ɫ���������ڱ���ˮ����CuO+H2 Cu+H2O
Cu+H2O
ʵ�鷴˼����������װ�ÿ��Բⶨ��������ж�������������������ȣ�Ҫ�ﵽ��һĿ�ģ�ʵ��С�����ⶨ����ֵ�ǣ��μӷ�Ӧ��п��������װ��B�������������ʴ�Ϊ��AB
�����������㿼���֪ʶ��Ƚ϶࣬�й�ʵ�鷽������ƺͶ�ʵ�鷽�����������п����ȵ�֮һ�����ʵ�鷽��ʱ��Ҫע�������ٵ�ҩƷ����ķ������������˻�ѧ����ʽ����д��Ҫע����ƽ����������Ҫ������ʵ�����У�
����⣺��������裺п��Ũ���ᷴӦ���IJ������ᣬ������ˮ��ʹ��ҺŨ�Ƚ��ͳ�ϡ����ʴ�Ϊ��п��Ũ���ᷴӦ���IJ������ᣬ������ˮ��ʹ��ҺŨ�Ƚ��ͳ�ϡ���ᣬ����Zn+H2SO4��ϡ��=ZnSO4+H2��
�������ϣ�������̼�Ļ�ѧ�����У�CO2����ˮ���������Һ��Ӧ�ȣ��ʴ�Ϊ������Һ��д�������ƻ��������ƣ�
ʵ����֤����1�������������ɿ�����Ⱦ��Bװ�õ������dz�ȥ�������ʴ�Ϊ����ȥ��������
��2���������л�ԭ�ԣ���������к�����������Eװ���й۲쵽�������ǣ�����ͭ�ɺ�ɫ��Ϊ��ɫ���������ڱ���ˮ��������������ͭ��Ӧ����ͭ��ˮ���ʴ�Ϊ������ͭ�ɺ�ɫ��Ϊ��ɫ���������ڱ���ˮ����CuO+H2
 Cu+H2O
Cu+H2Oʵ�鷴˼����������װ�ÿ��Բⶨ��������ж�������������������ȣ�Ҫ�ﵽ��һĿ�ģ�ʵ��С�����ⶨ����ֵ�ǣ��μӷ�Ӧ��п��������װ��B�������������ʴ�Ϊ��AB
�����������㿼���֪ʶ��Ƚ϶࣬�й�ʵ�鷽������ƺͶ�ʵ�鷽�����������п����ȵ�֮һ�����ʵ�鷽��ʱ��Ҫע�������ٵ�ҩƷ����ķ������������˻�ѧ����ʽ����д��Ҫע����ƽ����������Ҫ������ʵ�����У�

��ϰ��ϵ�д�
�����Ŀ
 21��ʵ��ⶨ��һ����Ϊȼ�ϵ�ú�к�1%��������úȼ�պ���ͷų�����������Ⱦ������ͬʱҲ���γ������ԭ��֮һ����������ÿ�궬��ȼúȡůʱ��Ϊl50�죬ÿ��ȼú��Լ��20000�֣���ô����������һ������������ŷŵĶ��������������
21��ʵ��ⶨ��һ����Ϊȼ�ϵ�ú�к�1%��������úȼ�պ���ͷų�����������Ⱦ������ͬʱҲ���γ������ԭ��֮һ����������ÿ�궬��ȼúȡůʱ��Ϊl50�죬ÿ��ȼú��Լ��20000�֣���ô����������һ������������ŷŵĶ��������������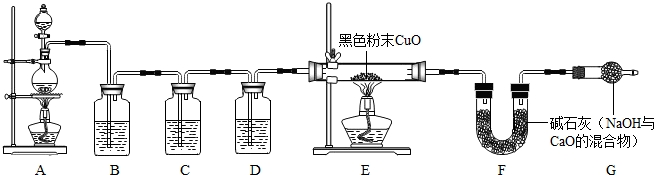
 ��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺
��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺