��Ŀ����
��ͥʳ������Ҫ�ɷ�ΪNa2CO3����������������NaCl����ͯͬѧΪ�ⶨʳ�������̼���Ƶĺ�������Ʋ�����������ʵ�飺�ٳ�ȡ�����Ʒ3.4g���ձ��У�����20mLˮ����������Ʒȫ���ܽ⣻
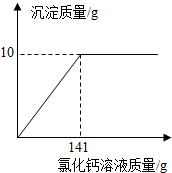
�������м���������CaCl2��Һ�����������ɳ���Ϊֹ��
�۹��˲������ó�������Ƶ�����Ϊ3.0g�������Dz����е�������ʧ����
����㣺ʳ�������Na2CO3�����������Ƕ��٣�����ȷ��0.1%��
���𰸡������������ɳ�������������Ʒ����������̼���ƺ��Ȼ��Ʒ�Ӧ�Ļ�ѧ����ʽ���Լ����ʳ�������̼���Ƶ�����������
����⣺��ʳ�������̼���Ƶ���������Ϊx��
Na2CO3+CaCl2=CaCO3��+2NaCl
106 100
3.4g×x 3.0g
 =
= ��x��93.5%
��x��93.5%
��ʳ�������̼���Ƶ���������Ϊ93.5%��
������������Ҫ���麬�������ʵĻ�ѧ����ʽ���㣬�ѶȽϴ�
����⣺��ʳ�������̼���Ƶ���������Ϊx��
Na2CO3+CaCl2=CaCO3��+2NaCl
106 100
3.4g×x 3.0g
 =
= ��x��93.5%
��x��93.5%��ʳ�������̼���Ƶ���������Ϊ93.5%��
������������Ҫ���麬�������ʵĻ�ѧ����ʽ���㣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
 ��2012?ɳ������ģ����ͥʳ������Ҫ�ɷ�ΪNa2CO3����������������NaCl��ij����̽��С���ͬѧΪ�ⶨʳ�������̼���Ƶ�������������Ʋ�����������ʵ�飺
��2012?ɳ������ģ����ͥʳ������Ҫ�ɷ�ΪNa2CO3����������������NaCl��ij����̽��С���ͬѧΪ�ⶨʳ�������̼���Ƶ�������������Ʋ�����������ʵ�飺