��Ŀ����
���û�ѧ֪ʶ�ش�
��1����ʯ�ҽ���ˢǽ�ں�����ڷ���һȼ�յ�ú¯����ʹǽ��Ѹ��Ӳ����ͬʱǽ���ϻ����һЩˮ�飬��ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
��2�����ú̿������ʵ�ضѻ������ܻ��������������¶����߶���ȼ���������ѻ���ú����ȼ��ʱ��������÷����� ��дһ�֣���
��3��Ϊ��ֹ�˵����⣬�����������е��� ��ѡ����ţ�
A���˵�ʹ�ú��ø�Ĩ������ B���˵�ʹ�ú�Ϳֲ���� C���˵�ʹ�ú�����ˮ����
��1����ʯ�ҽ���ˢǽ�ں�����ڷ���һȼ�յ�ú¯����ʹǽ��Ѹ��Ӳ����ͬʱǽ���ϻ����һЩˮ�飬��ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
��2�����ú̿������ʵ�ضѻ������ܻ��������������¶����߶���ȼ���������ѻ���ú����ȼ��ʱ��������÷����� ��дһ�֣���
��3��Ϊ��ֹ�˵����⣬�����������е��� ��ѡ����ţ�
A���˵�ʹ�ú��ø�Ĩ������ B���˵�ʹ�ú�Ϳֲ���� C���˵�ʹ�ú�����ˮ����
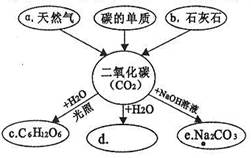
��1��CO2+Ca��OH��2==CaCO3��+H2O ��2�֣� ��2����ˮ����1�֣�
��3��AB��2�֣�ѡ��1����1�֣���ѡ��0�֣�
��3��AB��2�֣�ѡ��1����1�֣���ѡ��0�֣�
������Ҫ���պö�����̼�Ļ�ѧ���ʣ�����ѧ֪ʶ�ܸ��ݾ������ѡ�����ķ�������ѧ�����û�ѧ֪ʶ�����������ʶ���⣮
��𣺽⣺��1���������ƺͶ�����̼��Ӧ����̼��ƺ�ˮ����ѧ����ʽΪ��Ca��OH��2+CO2=CaCO3��+H2O����Ӳ����Ϊ����̼��ƣ�ͬʱǽ���ϻ����һЩˮ������Ϊ������ˮ��Ե�ʣ�
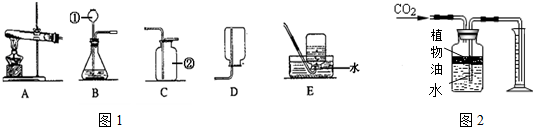
��2��ú�������������������������ų����������������������ʱ����������ȼ��ú����ȼ��ʱ��������÷����Ǹ���������������ɳ��ʯ�ȷ�������������������ˮ������ĭ��������𣬻����ˮú����H2��CO�Ļ�����壩��
��3�����ݷ�ֹ��������ķ����������������������з��������ǣ��Ӷ��ó���ȷ�Ľ��ۣ���������������Ǹ�����������ˮͬʱ�Ӵ���������Һ��������Һ������Һ�ܴٽ��������⣬��AB�Զ�C����
��Ϊ����1��Ca��OH��2+CO2=CaCO3��+H2O����2����ɳ�Ӹ��𣻣�3��AB��
��𣺽⣺��1���������ƺͶ�����̼��Ӧ����̼��ƺ�ˮ����ѧ����ʽΪ��Ca��OH��2+CO2=CaCO3��+H2O����Ӳ����Ϊ����̼��ƣ�ͬʱǽ���ϻ����һЩˮ������Ϊ������ˮ��Ե�ʣ�
��2��ú�������������������������ų����������������������ʱ����������ȼ��ú����ȼ��ʱ��������÷����Ǹ���������������ɳ��ʯ�ȷ�������������������ˮ������ĭ��������𣬻����ˮú����H2��CO�Ļ�����壩��
��3�����ݷ�ֹ��������ķ����������������������з��������ǣ��Ӷ��ó���ȷ�Ľ��ۣ���������������Ǹ�����������ˮͬʱ�Ӵ���������Һ��������Һ������Һ�ܴٽ��������⣬��AB�Զ�C����
��Ϊ����1��Ca��OH��2+CO2=CaCO3��+H2O����2����ɳ�Ӹ��𣻣�3��AB��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ