��Ŀ����
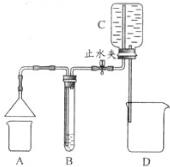 ��2010?��������ѧС��ͬѧ����ͼ��ʾװ�ã��г���������ȥ������2����Ȥʵ�飮ÿ��ʵ��ʱ����ֹˮ�У����ɼ�Cƿ�ڵ�ˮ����D�У�B���������ݳ���
��2010?��������ѧС��ͬѧ����ͼ��ʾװ�ã��г���������ȥ������2����Ȥʵ�飮ÿ��ʵ��ʱ����ֹˮ�У����ɼ�Cƿ�ڵ�ˮ����D�У�B���������ݳ�����1����A������ȼ�յ�����B����ɫ��Һ����ǣ�B�е��Լ���
����������Һ
����������Һ
������ȼ�����ɵ����屻����B�е�ԭ����Cƿ�ڵ�ˮ����D�У�ʹCƿ��ѹǿС�ڴ���ѹ��A�����屻����B��
Cƿ�ڵ�ˮ����D�У�ʹCƿ��ѹǿС�ڴ���ѹ��A�����屻����B��
����2����A������ij��Һ����B��ʢ����ɫ��Һ�ף�����ɫ����B��ʢ�к�ɫ��Һ�ң������ɫ���ҿ�����
���з�̪�ļ�����Һ
���з�̪�ļ�����Һ
�����A��B�������ʵ����ʽ����ұ�ɫ��ԭ��A�лӷ��������������ʽ���B�У���B�м������ʷ�����Ӧ��ʹ��Һ��ɫ��ȥ
A�лӷ��������������ʽ���B�У���B�м������ʷ�����Ӧ��ʹ��Һ��ɫ��ȥ
����������1����������ȼ�յ�������ͷ�Ӧ����������������֪ʶ�������屻����Bװ�õ�ԭ��
��2����ɫ��Һ�����ʯ����������Һ����ɫ����ɫ������кͽǶȷ�����
��2����ɫ��Һ�����ʯ����������Һ����ɫ����ɫ������кͽǶȷ�����
����⣺��1������ȼ�����ɶ�����̼��������̼��ʹ����ʯ��ˮ����ǣ�����ȼ�����ɵ������ܱ�����Bװ�õ�ԭ����Cװ���е�ˮ����Dƿ��ʹCƿ����ѹС�ڴ���ѹ�����屻ѹ��Bװ�ã�
��2���������Ʋ�Aװ������һ�ֻӷ����ᣬBװ���е���Һ���Ƿ�̪�ͼ���Һ�Ļ�����A�е���ӷ���������������Bװ�ú������кͷ�Ӧ������ɫ��ȥ��
�ʴ�Ϊ����1������������Һ�� Cƿ�ڵ�ˮ����D�У�ʹCƿ��ѹǿС�ڴ���ѹ��A�����屻����B�У�
��2�����з�̪�ļ�����Һ��A�лӷ��������������ʽ���B�У���B�м������ʷ�����Ӧ��ʹ��Һ��ɫ��ȥ��
��2���������Ʋ�Aװ������һ�ֻӷ����ᣬBװ���е���Һ���Ƿ�̪�ͼ���Һ�Ļ�����A�е���ӷ���������������Bװ�ú������кͷ�Ӧ������ɫ��ȥ��
�ʴ�Ϊ����1������������Һ�� Cƿ�ڵ�ˮ����D�У�ʹCƿ��ѹǿС�ڴ���ѹ��A�����屻����B�У�
��2�����з�̪�ļ�����Һ��A�лӷ��������������ʽ���B�У���B�м������ʷ�����Ӧ��ʹ��Һ��ɫ��ȥ��
�������������֪ʶ�е���ѹ�ĸı�͵��͵ķ�Ӧ�������ַ�����ʯ��ˮ����Ǿ��Ƕ�����̼���������Ʒ�Ӧ����Һ�ɺ�ɫ����ɫ�Ϳ��Ƿ�̪��Һ���кͷ�Ӧ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��Ĥ����������һ���ĵ����ظ�����LED���ϣ�����˵����ȷ���ǣ� ��
��Ĥ����������һ���ĵ����ظ�����LED���ϣ�����˵����ȷ���ǣ� ��

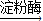 �����ǣ�C6H12O6��
�����ǣ�C6H12O6�� �Ҵ�+������̼
�Ҵ�+������̼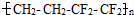 ��Ĥ����������һ���ĵ����ظ�����LED���ϣ�����˵����ȷ���ǣ� ��
��Ĥ����������һ���ĵ����ظ�����LED���ϣ�����˵����ȷ���ǣ� ��
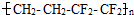 ��Ĥ����������һ���ĵ����ظ�����LED���ϣ�����˵����ȷ���ǣ� ��
��Ĥ����������һ���ĵ����ظ�����LED���ϣ�����˵����ȷ���ǣ� ��
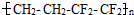 ��Ĥ����������һ���ĵ����ظ�����LED���ϣ�����˵����ȷ���ǣ� ��
��Ĥ����������һ���ĵ����ظ�����LED���ϣ�����˵����ȷ���ǣ� ��