��Ŀ����
����Ŀ������������ı��⣬���к�ˮռ������ˮ����96.53%���Ժ�ˮ���п�ѧ�Ĵ�������Ϊ�����ṩ��������IJ�Ʒ��
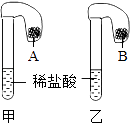
��1����ˮ������ѡ������������
��2�������Ǻ�ˮ��������Ҫ����֮һ��������ˮ������ˮ��������ȴ���õ��ߴ��ȵ�ˮ���ɴ˿��ж���������ѡ���������ѧ�����仯��
��3������ˮ����������ɲ����������ʵı���ʳ��ˮ��ͨ�������Ƶ��ռ�Ȼ�����Ʒ����ѧ��ӦΪ��2NaCl+2H2O=2NaOH+H2��+Cl2��������Ҫͨ������Ƶ��ռ�400�֣�������Ӧ����ʳ�ζ��ٶ֣�
���𰸡�
��1�������
��2������
��3���⣺������ʳ�ε�����Ϊx��
2NaCl+ | 2H2O | = | 2NaOH+ | H2��+ | Cl2�� |
117 | 80 | ||||
x | 400t |
![]() =
= ![]() ��
��
x=585t��
������Ҫͨ������Ƶ��ռ�400�֣�������Ӧ����ʳ��585t
���������⣺��1����ˮ�к���ˮ���Ȼ��Ƶ����ʣ����ڻ��� ���������2������ˮ������ˮ��������ȴ���õ��ߴ��ȵ�ˮ��������û�����������ʣ����������仯��
���������
�����㾫����������Ŀ����֪���������ø��ݻ�ѧ��Ӧ����ʽ�ļ���ʹ�����ͻ������б�����֪ʶ���Եõ�����Ĵ𰸣���Ҫ���ո����ʼ�������=ϵ������Է�������֮�ȣ�����������ͬһ��������ɵ�����.���������ʺͻ�����.��������ɶ���������ɵ����ʣ������Ҳ�����ɵ�����ɣ���������������һ�𣬾����ɵ�����ɵĻ�����֮����������������ֻ��������ϵ����ʣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�