��Ŀ����
�±�������������������Ӧ�¶�ʱ���ܽ�����ݣ��Լ��ڶ�Ӧ��������Һ����������Ļ�ѧʽ��
| ������ �� | A | B | |||||||
| �¶�[t���棩] | 0 | 10 | 20 | 30 | 40 | 50 | 70 | 80 | 90 |
| �ܽ��[S��g��] | 15.65 | 20.51 | 26.5 | 32.9 | 40.2 | 48.6 | 50.9 | 43.6 | 37.3 |
| ��Һ������ ����Ļ�ѧʽ | FeSO4?7H2O | FeSO4?H2O | |||||||

��2���ڽ���P������������Һ�У�������������Ϊ______�������С�������
��3�����ڼ��ŵ��ϡ��´���д�������¶����ݣ��Ա�ʾ���й�ϵʽ�ij�����[���У���ѧʽ�������ڵ���ĸ���壺��S���������壻��L������Һ�壮]
FeSO4?7H2O��S��
 FeSO4?H2O��S��+6H2O��L��
FeSO4?H2O��S��+6H2O��L��
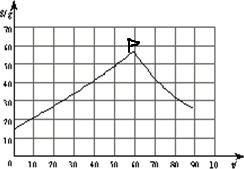
�⣺��1������������������ж���Һ�����������ʵ��ܽ�����ߣ�������ͼ��
��2��������������ݿ���֪��P��ʱ�����ʵ��ܽ��������Դ�ʱ������Һ����������Ҳ���
��3�����������ܽ��������Ȼ����õ���P������жϴ�ʱ����Ӧ���¶�ԼΪ60�棬��������¶�����FeSO4?7H2O��FeSO4?H2O����Һ���������ٽ�㣬���¶ȴ���60��ʱ����Һ��������������FeSO4?H2O�����¶ȵ���60��ʱ����Һ��������������FeSO4?7H2O��
��1��
��2�����
��3��

��������1��������������ʵ��ڲ�ͬ�¶��µ��ܽ����������ߣ��������ܽ�����ߣ�
��2����������ͼ����P���λ�����жϴ�ʱ���ʵ�����������
��3������P��������λ�����ж�FeSO4?7H2O��FeSO4?H2O�Ĵ���״̬��
�������������������ڲ�ͬ�¶��µ��ܽ���������ܽ�����������DZ���߱�����������֪���ܽ����������ʾ�����壮
��2��������������ݿ���֪��P��ʱ�����ʵ��ܽ��������Դ�ʱ������Һ����������Ҳ���
��3�����������ܽ��������Ȼ����õ���P������жϴ�ʱ����Ӧ���¶�ԼΪ60�棬��������¶�����FeSO4?7H2O��FeSO4?H2O����Һ���������ٽ�㣬���¶ȴ���60��ʱ����Һ��������������FeSO4?H2O�����¶ȵ���60��ʱ����Һ��������������FeSO4?7H2O��
��1��

��2�����
��3��

��������1��������������ʵ��ڲ�ͬ�¶��µ��ܽ����������ߣ��������ܽ�����ߣ�
��2����������ͼ����P���λ�����жϴ�ʱ���ʵ�����������
��3������P��������λ�����ж�FeSO4?7H2O��FeSO4?H2O�Ĵ���״̬��
�������������������ڲ�ͬ�¶��µ��ܽ���������ܽ�����������DZ���߱�����������֪���ܽ����������ʾ�����壮

��ϰ��ϵ�д�
 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
�����Ŀ
�±�������������������Ӧ�¶�ʱ���ܽ�����ݣ��Լ��ڶ�Ӧ��������Һ����������Ļ�ѧʽ��
��1�����������ϱ���������ݣ���������ڻ���A��B�����ܽ�����ߣ�����������A��B�����ƣ���A���ҡ�B������Ȼ����ֱ�����������ཻΪֹ���õ�����P��

��2���ڽ���P������������Һ�У�������������Ϊ______�������С�������
��3�����ڼ��ŵ��ϡ��´���д�������¶����ݣ��Ա�ʾ���й�ϵʽ�ij�����[���У���ѧʽ�������ڵ���ĸ���壺��S���������壻��L������Һ�壮]
FeSO4?7H2O��S�� FeSO4?H2O��S��+6H2O��L��
FeSO4?H2O��S��+6H2O��L��
| �� �� | A | B | |||||||
| �¶�[t���棩] | 10 | 20 | 30 | 40 | 50 | 70 | 80 | 90 | |
| �ܽ��[S��g��] | 15.65 | 20.51 | 26.5 | 32.9 | 40.2 | 48.6 | 50.9 | 43.6 | 37.3 |
| ��Һ������ ����Ļ�ѧʽ | FeSO4?7H2O | FeSO4?H2O | |||||||

��2���ڽ���P������������Һ�У�������������Ϊ______�������С�������
��3�����ڼ��ŵ��ϡ��´���д�������¶����ݣ��Ա�ʾ���й�ϵʽ�ij�����[���У���ѧʽ�������ڵ���ĸ���壺��S���������壻��L������Һ�壮]
FeSO4?7H2O��S��
 FeSO4?H2O��S��+6H2O��L��
FeSO4?H2O��S��+6H2O��L��

 FeSO4?H2O��S��+6H2O��L��
FeSO4?H2O��S��+6H2O��L��