��Ŀ����
�ش��������⣮
��1���������Ҫ���дһ����ѧ��Ӧ����ʽ��
����þ�μӵĻ��Ϸ�Ӧ______
�����������������ɵķֽⷴӦ______
���н������ɵ��û���Ӧ______
����ˮ���ɵĸ��ֽⷴӦ______
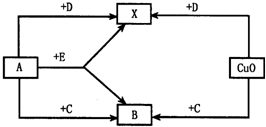
��2��A��B��C��D��E�dz��л�ѧ�г�����������ɫ���壬���������ǵ��ʣ��������ǻ��������֮������ͼ��ʾ��ת����ϵ��ͼ��δע����Ӧ������X�ڳ����²������壮��
��д������������Ļ�ѧʽ��
A______��B______��C______��D______��E______��
�����������������У����������A�����Ԫ�ص��ǣ��ѧʽ��______��
�⣺��1������þ�μӵĻ��Ϸ�Ӧ����дþ��������������þ���ʴ�Ϊ��2Mg+O2 2MgO��
2MgO��
�����������������ɵķֽⷴӦ����д��ʽ̼��ͭ�ֽ���������ͭ��ˮ�Ͷ�����̼���ʴ�Ϊ��Cu2��OH��2CO3 2CuO+H2O+CO2����
2CuO+H2O+CO2����
���н������ɵ��û���Ӧ����д��������ͭ����ͭ�������������ʴ�Ϊ��Fe+CuSO4=Cu+FeSO4
����ˮ���ɵĸ��ֽⷴӦ����д����кͣ��ʴ�Ϊ��HCl+NaOH=NaCl+H2O
��2��������ͭ����ɫ�����巴Ӧ������ˮ�����Ʋ��D��������������ͭ������C��Ӧ������B�������Ʋ��C��һ����̼��B�Ƕ�����̼������A��һ����̼��Ӧ�����ɶ�����̼������֪��A����������������������Ӧ����ˮ������������E��Ӧ����ˮ�Ͷ�����̼������֪��E���Ǽ��飬
�ʴ�Ϊ��A O2��B CO2��C CO��D H2��E CH4��
�����������������У�����в�����Ԫ�ص��� H2��CH4���ʴ�Ϊ��H2��CH4��
��������1����Ҫ����д��ѧ����ʽʱ����Ҫȷ����Ӧԭ�������ݷ�Ӧ��������P��Ӧ�������������غ㶨����ɻ�ѧ����ʽ����д��
��2��������������������������������������ƽ������Ժ����������ó����ۣ��ɴ�����ͭ��D��Ӧ������ˮ����ͻ�ƣ�Ȼ���ϳ�������ɫ��������ʡ����ʵļ�������ʵ��ת����ϵ�����Եó�������ȷ�Ĵ𰸣�
��������д��ѧ����ʽҪע�����ʵĻ�ѧʽ����ƽ����Ӧ�������������ɷ��ϻ�������ϵȣ������ͼ�ƶ���ʱ�ؼ��ҳ����е�ͻ�Ƶ㣬�����ͻ�Ƶ�������ͭ������D��Ӧ����ˮ��
 2MgO��
2MgO�������������������ɵķֽⷴӦ����д��ʽ̼��ͭ�ֽ���������ͭ��ˮ�Ͷ�����̼���ʴ�Ϊ��Cu2��OH��2CO3
 2CuO+H2O+CO2����
2CuO+H2O+CO2�������н������ɵ��û���Ӧ����д��������ͭ����ͭ�������������ʴ�Ϊ��Fe+CuSO4=Cu+FeSO4
����ˮ���ɵĸ��ֽⷴӦ����д����кͣ��ʴ�Ϊ��HCl+NaOH=NaCl+H2O
��2��������ͭ����ɫ�����巴Ӧ������ˮ�����Ʋ��D��������������ͭ������C��Ӧ������B�������Ʋ��C��һ����̼��B�Ƕ�����̼������A��һ����̼��Ӧ�����ɶ�����̼������֪��A����������������������Ӧ����ˮ������������E��Ӧ����ˮ�Ͷ�����̼������֪��E���Ǽ��飬
�ʴ�Ϊ��A O2��B CO2��C CO��D H2��E CH4��
�����������������У�����в�����Ԫ�ص��� H2��CH4���ʴ�Ϊ��H2��CH4��
��������1����Ҫ����д��ѧ����ʽʱ����Ҫȷ����Ӧԭ�������ݷ�Ӧ��������P��Ӧ�������������غ㶨����ɻ�ѧ����ʽ����д��
��2��������������������������������������ƽ������Ժ����������ó����ۣ��ɴ�����ͭ��D��Ӧ������ˮ����ͻ�ƣ�Ȼ���ϳ�������ɫ��������ʡ����ʵļ�������ʵ��ת����ϵ�����Եó�������ȷ�Ĵ𰸣�
��������д��ѧ����ʽҪע�����ʵĻ�ѧʽ����ƽ����Ӧ�������������ɷ��ϻ�������ϵȣ������ͼ�ƶ���ʱ�ؼ��ҳ����е�ͻ�Ƶ㣬�����ͻ�Ƶ�������ͭ������D��Ӧ����ˮ��

��ϰ��ϵ�д�
 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
�����Ŀ
��ͨ��ͭ����ͭ��п��ɣ��㷺���������ġ��ܲĵȣ�Ҳ���������е�����Ϊ�ⶨ��ͭ��ͭ������������ȡ��Ʒ10g�����Ĵ������м���ϡ����ʹ֮��ַ�Ӧ��ʵ�����ݼ�¼���±���
����������ݣ��ش��������⣺
��1����ͭ��ͭ����������Ϊ
��2��ǡ����ȫ��Ӧʱ����Һ�����ʵ����������ǣ�
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ����ϡ���������/g | 10 | 10 | 10 | 10 |
| ʣ����������/g | 8.7 | 7.4 | 7 | 7 |
��1����ͭ��ͭ����������Ϊ
��2��ǡ����ȫ��Ӧʱ����Һ�����ʵ����������ǣ�
��Һ�����������ϢϢ��أ���Һ���������ճ�����ͻ�ѧʵ���еij����������±���������Һ�Ͱ�ˮ���ܶ��������ʵ������������ձ���20�棩��
����ϸ������ش��������⣺
��1��20��ʱ��������Һ�����ʵ���������������������Һ���ܶ��� �����������䣩����ˮ���ܶ��� ��������С�䣩
��2��ȡ12%��������Һ100g���Ƴ�6%����Һ����100g12%��������Һ�м�ˮ������
Ӧ 100g������ڡ�С�ڻ���ڣ���
��3����100g24%�İ�ˮ�м���100gˮ��ҡ�ȣ���Һ����� mL��������0.1����
| ��Һ�����ʵ���������/% | 4 | 12 | 16 | 24 | 28 |
| ������Һ���ܶ�/g/mL | 1.02 | 1.08 | 1.11 | 1.17 | 1.20 |
| ��ˮ���ܶ�/g/mL | 0.98 | 0.95 | 0.94 | 0.91 | 0.90 |
��1��20��ʱ��������Һ�����ʵ���������������������Һ���ܶ���
��2��ȡ12%��������Һ100g���Ƴ�6%����Һ����100g12%��������Һ�м�ˮ������
Ӧ
��3����100g24%�İ�ˮ�м���100gˮ��ҡ�ȣ���Һ�����
