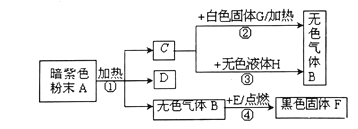
��Ŀ����
����Ŀ���ҹ�ú̿��Դ�ḻ��Ŀǰ���dz��˽�ú��Ϊȼ���⣬������Ҫ�Ļ���ԭ�ϡ���ҵ����ú�Ϳ���Ϊԭ���������أ�CO(NH2)2]��һ���������£�
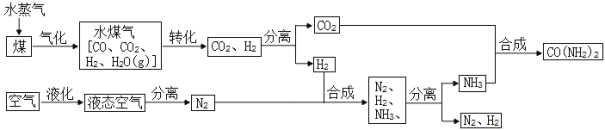
��1����Һ̬�����з����N2�Ĺ�������____________�����������ѧ�����仯��
��2����ú��ˮ������Ӧǰ���Ƚ�ú���飬��������Ŀ����___________________________��
��3�����������кϳ����ص�ͬʱ����ˮ���ɣ��÷�Ӧ�ķ��ű���ʽʽΪ________________��
��4��ʵ�������У�N2��H2������ȫ����ΪNH3�����������п��Ի����õ�������_______________��
���𰸡����� ����ú��ˮ�ĽӴ������ʹ��Ӧ�������� CO2+NH3![]() CO��NH2��2+H2O H2O��N2��H2
CO��NH2��2+H2O H2O��N2��H2
��������
��1����Һ̬�����з����N2�Ĺ�����û�����������ɣ����������仯��
��2����ú��ˮ������Ӧǰ���Ƚ�ú���飬��������Ŀ���ǣ�����ú��ˮ�ĽӴ������ʹ��Ӧ�������֣�
��3��������̼�Ͱ�����Ӧ�������غ�ˮ����Ӧ�ķ��ű���ʽΪ��CO2+NH3![]() CO��NH2��2+H2O��
CO��NH2��2+H2O��
��4�����Ƿ�Ӧ��Ҳ������������ʿ���ѭ��ʹ�ã��������������п��Ի����õ������У�H2O��N2��H2��