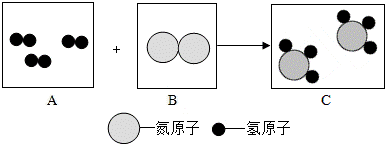
��Ŀ����
��8�֣����������������������Ź㷺��Ӧ�á�
��1�����ڿ����о��кܺõĿ���ʴ���ܣ�ԭ���ǣ��û�ѧ����ʽ��ʾ��
��2�������ڼ䣬С��������г�����һƥ���ɫ�ġ�������������װ��Ʒ��С����������пͭ�Ͻ�����ǻƽ�Ϊ�˱����α����ѡ������ ���йصĻ�ѧ����ʽ ___
A������ B�������� C���Ȼ�þ
��3����ҵ����һ����̼���»�ԭ980t��������80%�ij�����ʯ����������صĻ�ѧ����ʽ ������������98%������ t��
��4��������ͭ������п��ϡ����Ļ����Һ�У�����һ���������ۣ�ʹ֮��ַ�Ӧ��������ʣ�࣬���ˣ�����Һ�д��ڴ����������� �������ӷ��ţ�
��1��4Al+3O2====2Al2O3��1�֣�����2�� A ��1�֣���Zn+H2SO4 ==ZnSO4 +H2����1�֣���
��3��3CO + Fe2O3����2Fe + 3CO2 ��1�֣�©д��Ӧ�������²����� �� 560t��2�֣���
��4��Fe2+��Zn2+ ��2�֣� ���һ����1�֣����ӷ���д�������֣�
����

��ϰ��ϵ�д�
�����Ŀ