��Ŀ����
����Ŀ��ij�о���ѧϰС��ԡ�SO2�ܷ���H2O��Ӧ�����ᡱ�Ŀ��չ̽�����������Ϻ��֪��
��SO2��������һ����ɫ���壬������ˮ��
������ʹ��ɫ��ʯ����ֽ��ɺ�ɫ��
��SO2�ж����������������ͼ��ʾװ�ý���ʵ�飮
����������ǵ�̽������ش��й����⣺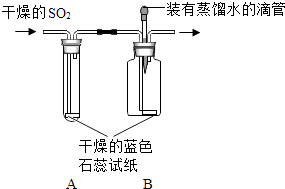
��1��ʵ������У�Aװ������ɫʯ����ֽ����ɫʼ��û�б仯��Aװ�õ������ǣ���
��2����ͨ��SO2֮ǰ��Bװ���н�ͷ�ι��ڵ�����ˮ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯���ɴ˵ó��Ľ����� �� ����SO2ͨ��ʱ������ʪ����ɫʯ����ֽ��죬������˵����
��3��ʵ�鷽���У���һ�����Ե���©�����������ָ������֮�� ��
���𰸡�
��1��֤��SO2����ʹ��ɫʯ����ֽ���
��2��֤��ˮ����ʹ��ɫʯ����ֽ��죨��֤��ˮû�����ԣ�,SO2��H2O��Ӧ������
��3��β��û�д�������Ӧ��һ��β������װ�ã�
���������⣺��1��Aװ������ɫʯ����ֽ����ɫʼ��û�б仯��˵������Ķ���������ʹ��ʯ����ֽ��ɫ�����Դ��ǣ�֤��SO2����ʹ��ɫʯ����ֽ��죨2����ͨ��SO2֮ǰ��Bװ���н�ͷ�ι��ڵ�����ˮ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯��˵��ˮ����ʹ���ɫ������SO2ͨ��ʱ������ʪ����ɫʯ����ֽ��죬˵������������ˮ��Ӧ�������ᣮ���Դ��ǣ�֤��ˮ����ʹ��ɫʯ����ֽ��죨��֤��ˮû�����ԣ���SO2��H2O��Ӧ�����ᣮ��3���������Ͽ�֪��SO2�ж�������Ⱦ��������˶�β��Ҫ���д��������Դ��ǣ�β��û�д�������Ӧ��һ��β������װ�ã�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���±���Ca(OH)2��NaOH���ܽ�����ݣ���ش��������⣺
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
�ܽ��/g | Ca(OH)2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 |
NaOH | 31 | 91 | 111 | 129 | 313 | 336 | |
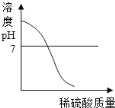
(1)�����ϱ����ݣ�����Ca(OH)2��NaOH���ܽ�����ߣ���ͼ��ʾ�ܱ�ʾNaOH�ܽ�����ߵ���___________(��A��B)��
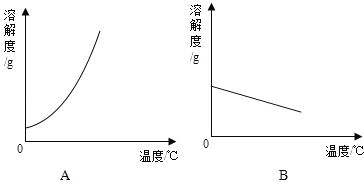
(2)Ҫ���һƿ�ӽ����͵�Ca(OH)2��Һ��ɱ�����Һ�������ʩ�У��ټ����������ƣ��������¶ȣ��۽����¶ȣ��ܼ���ˮ��������ˮ���ٻָ���ԭ�¶ȣ�������ʯ�ң����д�ʩ��ȷ����__________��
A���ڢܢ� B���ۢ� C���٢ۢݢ� D���٢ڢݢ�
(3)60��ʱ��229g����NaOH��Һ������50gˮ���ٽ��µ�20�棬������NaOH���������Ϊ___________��
(4)����20��ʱCa(OH)2�ı�����Һ(����Һ)�������м���һ����CaO��õ�����Һ(����Һ)����ʱ��Һ�����ʵ��������� ��___________��(��������������������=��)��
(5)����60��ʱ��Ca(OH)2��NaOH�������ʵı�����Һ����Ҫ�õ��ϴ�����NaOH���壬Ӧ��ȡ������������___________��
(6)20��ʱ�����ⶨNaOH��Һ��pH�����Ƚ�pH��ֽ������ˮ��ʪ���ٽ��вⶨ����������Һ��pH___________(����ƫ��������ƫС����������Ӱ����)��