��Ŀ����
��������ȤС��ͬѧ�Ա�¶�ڿ������������ƹ����̽������ش��������⣺
��������⣩
���������ƹ�����û�б��ʣ�
������ʵ�飩
��ͬѧȡ�����������Թ�����������ˮ�ܽ⣬������������Ȼ�����Һ���۲쵽__________��֤���������ƹ����Ѿ�����̼���ơ�
��������⣩
��γ�ȥ�������ƹ����е����ʣ��õ����������������أ�
������ʵ�飩
��ͬѧ�Ը��������ƹ�������ᴿ�������²������̽���ʵ�飺

��ʵ�������
��1������ڵ���Ҫ����������__________��������еķ�Ӧ�Ļ�ѧ����ʽ��__________��
��2��������м����Թ�������������Ŀ����__________��
��3��Ϊ��ô������������ƣ�������������ľ������������Ũ����__________��ѡ������ᾧ�����½ᾧ���������ˡ�
��4����ͬѧ��Ϊ��ͬѧ�����е�������������ˮ����Ҫʹ�ýϴ�����ˮ����������Ѷȣ�������_______�Լ����档
��ʵ����չ��
��βⶨ���õ��ռ���Ʒ���������Ƶ�����������
��һƿ���õ��ռ��г�ȡ20g�������ʣ������������Ϊ19g����ȫ����ˮ�����100g��Ʒ��Һ����ȡһ�����ʵ������������Ȼ�����Һ����Ʒ��Һ��ϣ���ַ�Ӧ��õ������ʾ�����ݡ�
��Ŀ�ʹ��� | ��1�� | ��2�� | ��3�� | ��4�� |
��Ʒ��Һ������g�� | 10 | 20 | 30 | 40 |
�Ȼ�����Һ������g�� | 10 | 15 | 15 | 30 |
����������������g�� | 1.97 | 3.94 | 3.94 | X |
���е�__________�η�Ӧǡ����ȫ����
��5���������Ʒ���������Ƶ�����������__________����д���������̣�
��1��С�ú�Сͮ�ڰ�����ʦ����ҩƷʱ����������ƿʧȥ��ǩ����Һ��ֻ֪�����Ƿֱ�������������Һ������ͭ��Һ��̼������Һ��ϡ���ᡣС�úܿ���жό�����ƿ������ͭ��Һ��Сͮ������������Һ�ֱ��ų�A��B��C��Ȼ��������Ͻ�������ʵ�飺
ʵ�� | A+B | B+C | A+C |
���� | ���������� | �а�ɫ�������� | ��������� |
�ݴˣ�Сͮ�ܿ�ͷֱ��������δ֪��Һ���ش��������⣺
��С���жϳ�����ͭ��Һ��������_________________��
��A��B��Ӧ�Ļ���������___________��
��C�Ļ�ѧʽ��___________��
��2����ͼ��Fe���仯����Ļ��ϼۡ�����������άͼ��
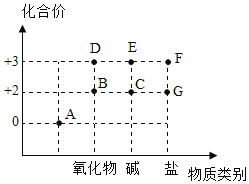
��A�����������������___________�����������ʡ�����
��C�ڳ�ʪ�Ŀ����к����������Ϸ�Ӧ���E���÷�Ӧ�Ļ�ѧ����ʽ��_________________________��
����ͼ������ȷ��ӳ��Ӧ�仯��ϵ����
|
|
|
|
A��һ�����Ķ��������м������������Һ | B����һ�����ĸ�����ع��� | C��һ���� AgNO3�� Cu(NO3)2 �Ļ����Һ�м������� | D�� NaOH ��Һ�еμ�ϡ���������� |
A.A B.B C.C D.D





