��Ŀ����
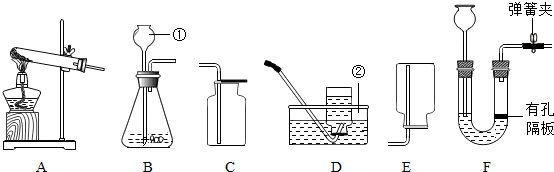
ijͬѧ������һ����������������̼������Һ���ⶨ�����ȣ�����������ͼ��ʾ��ʵ�������
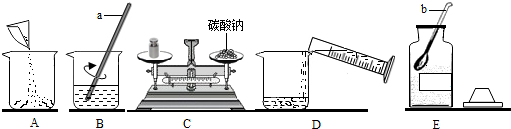
��1������д������������ƣ�a ��b ���ܽ�����õ�������a���������� ��
��2������ʵ�����ȷ����˳��Ϊ ������ĸ����������һ�������д�����˵����ȷ�IJ�������Ϊ ��
��3������50g������������Ϊ10%��̼������Һ����ҪNaCl g��ˮ mL�������ᵼ��������Һ��������������ƫС��ѡ���� ������ţ�
A�� ̼�����к������� B������ʱʹ�������������
C������Ͳ��ȡˮʱ�����Ӷ��� D��������ɺ���ȡҺ��ʱ�в��ֽ���
��4���ⶨ��������Һ������ʱ���Ƚ�pH��ֽ������ˮʪ���ٽ��вⶨ��������Һ��pHֵ ��ѡ�ƫ����ƫС������Ӱ�족����������ȷ�IJ��������ⶨ����������Һ��PH��7����̼������Һ�� �ԣ�ѡ��ᡱ��������С�����

��1������д������������ƣ�a
��2������ʵ�����ȷ����˳��Ϊ
��3������50g������������Ϊ10%��̼������Һ����ҪNaCl
A�� ̼�����к������� B������ʱʹ�������������
C������Ͳ��ȡˮʱ�����Ӷ��� D��������ɺ���ȡҺ��ʱ�в��ֽ���
��4���ⶨ��������Һ������ʱ���Ƚ�pH��ֽ������ˮʪ���ٽ��вⶨ��������Һ��pHֵ
���㣺һ������������������Һ������,������-������ƽ,��Һ�����Ȳⶨ
ר�⣺��Һ����Һ���ܽ��
��������1�����ݳ����Ļ�ѧ���������ƺ��ܽ�ʱ��������������ý��з������
��2����������������������һ������Һ�Ļ��������������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ����з�����ɣ�
��3��������������=��Һ���������������������ܼ�����=��Һ����-������������������������С�����������������ƫС���ܼ�����ƫ���Է��������������������������ԭ����з������
��4��������ˮʪ��pH��ֽ������ϡ���˴�����Һ��ʹ̼������Һ�ļ��Լ������ݴ˽��з������
��2����������������������һ������Һ�Ļ��������������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ����з�����ɣ�
��3��������������=��Һ���������������������ܼ�����=��Һ����-������������������������С�����������������ƫС���ܼ�����ƫ���Է��������������������������ԭ����з������
��4��������ˮʪ��pH��ֽ������ϡ���˴�����Һ��ʹ̼������Һ�ļ��Լ������ݴ˽��з������
����⣺��1������a�Dz�����������b��ҩ�ף�
��2������һ����������������̼������Һ�����ȼ���������Һ����̼���ƺ�ˮ���������ٳ��������̼���ƺ���ȡˮ���������ܽ⣬����ʵ�����ȷ����˳��ΪECADB��������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ��ͼ�г���ʱҩƷ�������λ�÷ŷ��ˣ�
��3������̼���Ƶ�����Ϊ50g��10%=5g������ˮ������Ϊ50g-5g=45g����45mL��
A���Ȼ����������ʣ��ᵼ�³������Ȼ��Ƶ�����ƫС����ʹ������������ƫС����ѡ����ȷ��
B������ʱʹ������������룬������Ȼ��Ƶ��������ӣ��ᵼ��������Һ������������ƫ��ѡ�����
C������Ͳ��ȡˮʱ������Һ�棬������ʵ��Һ�����С�������ʵ����ȡ��ˮ�����ƫ����ʹ������������ƫС����ѡ����ȷ��
D����Һ���о�һ�ԣ�������ɺ���ȡҺ��ʱ�в��ֽ��������������������䣬��ѡ�����
��4��̼������Һ�Լ��ԣ�����Һ��pH����7ʱ���ʼ��ԣ���pHԽ����Խǿ��������ˮʪ��pH��ֽ������ϡ���˴�����Һ��ʹ̼������Һ�ļ��Լ������ⶨ���ƫС��
�ʴ�Ϊ����1����������ҩ�ף�����ӿ��ܽ����ʣ�
��2��ECADB��C��Ӧ�������룻
��3��5��45��AC��
��4��ƫС���
��2������һ����������������̼������Һ�����ȼ���������Һ����̼���ƺ�ˮ���������ٳ��������̼���ƺ���ȡˮ���������ܽ⣬����ʵ�����ȷ����˳��ΪECADB��������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ��ͼ�г���ʱҩƷ�������λ�÷ŷ��ˣ�
��3������̼���Ƶ�����Ϊ50g��10%=5g������ˮ������Ϊ50g-5g=45g����45mL��
A���Ȼ����������ʣ��ᵼ�³������Ȼ��Ƶ�����ƫС����ʹ������������ƫС����ѡ����ȷ��
B������ʱʹ������������룬������Ȼ��Ƶ��������ӣ��ᵼ��������Һ������������ƫ��ѡ�����
C������Ͳ��ȡˮʱ������Һ�棬������ʵ��Һ�����С�������ʵ����ȡ��ˮ�����ƫ����ʹ������������ƫС����ѡ����ȷ��
D����Һ���о�һ�ԣ�������ɺ���ȡҺ��ʱ�в��ֽ��������������������䣬��ѡ�����
��4��̼������Һ�Լ��ԣ�����Һ��pH����7ʱ���ʼ��ԣ���pHԽ����Խǿ��������ˮʪ��pH��ֽ������ϡ���˴�����Һ��ʹ̼������Һ�ļ��Լ������ⶨ���ƫС��
�ʴ�Ϊ����1����������ҩ�ף�����ӿ��ܽ����ʣ�
��2��ECADB��C��Ӧ�������룻
��3��5��45��AC��
��4��ƫС���
�����������ѶȲ�����ȷ����һ������������������Һʵ�鲽�衢ע�����pH��ֽ��ʹ�÷���������ȷ�����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
ijͬѧ����ͼװ�ö������غ㶨�ɽ���ʵ��̽��������˵������ȷ���ǣ�������
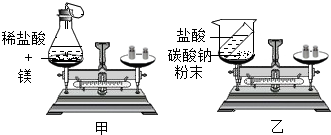

| A����װ�������ɵ������ܶȺ�С���������ʹ�����ĸ���ʹ��ƽ��ƽ�� |
| B����װ�������ɵĶ�����̼�����ݳ���ʹ��ƽ��ƽ�� |
| C����װ���з�Ӧ�Ļ�ѧ����ʽΪ2HCl+Na2CO3�T2NaCl+H2O+CO2�� |
| D�����������ɵķ�Ӧ������������֤�����غ㶨�� |
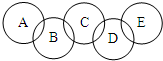 ijУ�Ļ�ѧ��ȤС��������һ�滯ѧ�廷�죬ͼ��A��B��C��D��E����������������������ϡ���ᡢ����������Һ�е�һ�����ʣ����������ʼ���һ�������¶��ܷ�����Ӧ�������������ʼ䲻������Ӧ����A����Է�������С��E�ģ�����д���пհף�
ijУ�Ļ�ѧ��ȤС��������һ�滯ѧ�廷�죬ͼ��A��B��C��D��E����������������������ϡ���ᡢ����������Һ�е�һ�����ʣ����������ʼ���һ�������¶��ܷ�����Ӧ�������������ʼ䲻������Ӧ����A����Է�������С��E�ģ�����д���пհף� ʵ���ҿ����գ�ij��ѧ��ȤС���ͬѧ����ʦ��ָ���£���Ƴ�����ʵ��װ�ö�̼������ͭ��Ӧ���ɵ�������������̽������ش��й����⣺
ʵ���ҿ����գ�ij��ѧ��ȤС���ͬѧ����ʦ��ָ���£���Ƴ�����ʵ��װ�ö�̼������ͭ��Ӧ���ɵ�������������̽������ش��й����⣺