��Ŀ����
����Ŀ����֪ij������Ħ������CaCO3�� SiO2��������ˮ�Ҳ���ϡ���ᷴӦ����Ϊ������Ħ�����������е�������������������ʵ�飺
��ȡ20g������Ʒ���ձ��У���ˮ����ܽ⡢���˵õ�Ħ������
����10����ϡ����ε������õ�Ħ�����С�
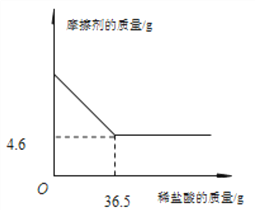
��Ħ���������ϡ�����������ϵ��ͼ��ʾ��
�Լ��㣺
��1����Ħ������CaCO3�����ʵ����Ƕ��٣�____________________
��2����������Ħ���������������Ƕ��٣�__________________
���𰸡� 0.05mol 48%
��������CaCO3�� SiO2�Ļ�����У�̼����������ᷴӦ�����ɿ��������ʣ���������������Ӧ��ʣ��Ĺ����Ƕ������裬������Ϊ4.6g����ͼ��֪����Ӧ��������Һ36.5g,�����Ȼ��������Ϊ3.65g�����ʵ���Ϊ0.1mol
Ħ������CaCO3�����ʵ���Ϊx
CaCO3 +2HCl ==CaCl2+ CO2�� + H2O
1 2
x 0.1mol
1/x=2/0.1mol x=0.05mol
������̼��Ƶ�����Ϊ0.05mol��100g/mol=5g, ��������Ħ���������������ǣ�5g+4.6g����20g��100%=48%
����

��ϰ��ϵ�д�
�����Ŀ