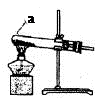��Ŀ����
ijͬѧ����֤�������ǵ���Ҫ�ɷ���̼���Σ����ռ����������塱��ʵ�飮��������·�������ʵ�飺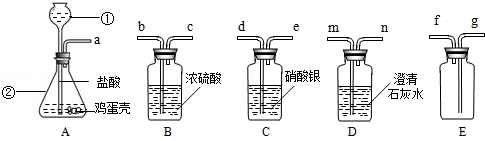
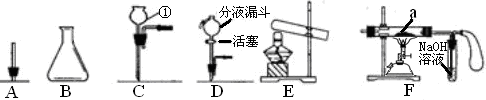
��1��д���������������ƣ��� ���� ��
��2������A��������� ��
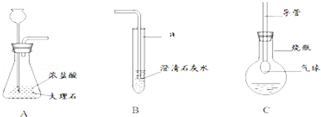
��3������������ѧ��֪ʶ������ΪAװ�ú� װ������������ʲô����ʱ������˵�������ǵ���Ҫ�ɷ���̼���Σ� д����װ���з�����Ӧ�Ļ�ѧ����ʽ�� ��
��4����ͬѧ��Ҫ�ռ�һƿ�����ĸ����壬��һ����֤�������ʣ���������װ�õ�˳���ǣ�����д���ӿ���ĸ�� �����У�Cװ�õ������ǣ� ��д���йط�Ӧ�Ļ�ѧ����ʽ�� ��
��5��д����Aװ�û�������ȡ���ճ�������һ�ֳ�������Ļ�ѧ��Ӧ����ʽ�� ����˵������һ����Ҫ��; ��
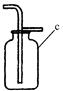
��1���ٳ���©��������ƿ
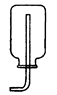
��2�������Ǹ���Һ���ϣ����������ݲ������������ܽ�
��3��D Dװ���г���ʯ��ˮ����ǣ�CO2+Ca��OH��2=CaCO3��+H2O
��4��a��d��e��b��c��g ����ȥCO2�л��е�HCl���� ��AgNO3+HCl=AgCl��+HNO3
��5��2H2O2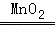 2H2O+O2�� ����������
2H2O+O2�� ����������
���������������1��ʶ���������ʴ�Ϊ���ٳ���©������ƿ��
��2��̼��ƺ����ᷴӦ�����Ȼ��ƺͶ�����̼��ˮ������̼��ƹ������٣�ͬʱ������ð�����ʴ�Ϊ�������Ǹ���Һ���ϣ����������ݲ������������ܽ⣮
��3��̼���κ����ᷴӦ���ɶ�����̼��������̼����ͨ�����ʯ��ˮ��ʯ��ˮ����ǣ��ʴ�Ϊ��D��Dװ���г���ʯ��ˮ����ǣ�CO2+Ca��OH��2=CaCO3��+H2O��
��4��̼��ƺ����ᷴӦ���ɶ�����̼���壬ͬʱ�����Ȼ����ˮ����������Ҫ��ȥ�Ȼ����ˮ�������Ȼ������������Ӧ�����Ȼ������������ᣬŨ���������ˮ�ԣ��������ڸ������壮�ʴ�Ϊ��a��d��e��b��c��g����ȥCO2�л��е�HCl���壬AgNO3+HCl=AgCl��+HNO3��
��5��Aװ�����ڹ����Һ�岻������ȡ���壬�ʴ�Ϊ��2H2O2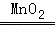 2H2O+O2������������
2H2O+O2������������
���㣺��������ķ���װ�ú��ռ�װ����ѡȡ�������������������ƺ�ѡ�ã���д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����ͼ�ش����⣺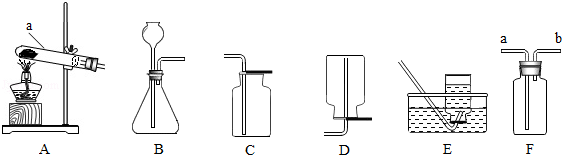
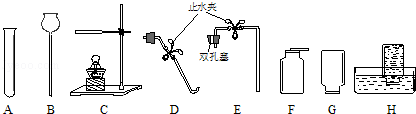
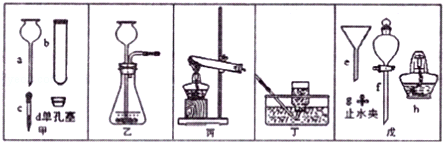
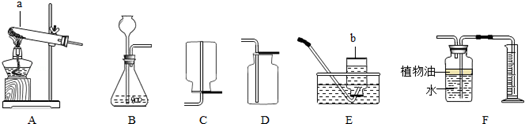
��l��ͼ��a���������� ��
��2���ø��������ȡ�����Ļ�ѧ����ʽΪ ���ռ�������ѡ�õ�װ���� ������ĸ����ͬ����
��3���ô���ʯ��ϡ������ȡ������̼ʱ����ѡ�õķ���װ���� ����Ӧ�Ļ�ѧ����ʽΪ ��
��4����ͼF��ʾװ���ж�����;������˵����ȷ���� ��
| A����ȥ�����е�ˮ��������ƿ��ʢ��Ũ���� |
| B�����������̼���塪��ƿ��ʢ������������Һ |
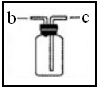
| C�����ſ������ռ��������������װ�õ�b��ͨ�� |
| D������ˮ���ռ���������ƿ����װ��ˮ�������b��ͨ�룮 |