��Ŀ����
Ԫ�����ڱ��ǻ�ѧѧϰ���о�����Ҫ���ߡ���ͼ��Ԫ�����ڱ���һ���֣�Ԫ��������ʡ�ԣ���
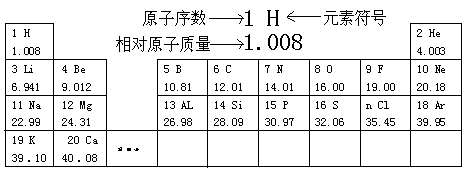
�����ݸñ���Ϣ�ش����⡣
(1)����14��Ԫ�ص������� �������к�������Ԫ�ص�ԭ�Ӻ����� �����ӣ�
(2)�����Ķ��ϱ���ԭ������Ϊ ��Ԫ�ط��Ŵ���Ӧ��д�� ��
(3) ��ϸ�۲��ϱ������õ�ijЩ���ɣ����Ը�����ó��Ĺ����Ʋ�ClԪ�ص�ԭ������n����ֵ�� ����д���������� ��
(4)ԭ������Ϊ9��Ԫ�ص�ԭ�ӵõ����Ӻ��γ�-1�۵����ӣ������ӷ����� ��
(5)���ñ��е�Ԫ�ط���д������Ҫ��Ļ�ѧ��Ӧ����ʽ��һ�����н����μӵĻ��Ϸ�Ӧ�� �����ַǽ������ʷ����Ļ��Ϸ�Ӧ�� ��
(1)����14��Ԫ�ص������� �������к�������Ԫ�ص�ԭ�Ӻ����� �����ӣ�
(2)�����Ķ��ϱ���ԭ������Ϊ ��Ԫ�ط��Ŵ���Ӧ��д�� ��
(3) ��ϸ�۲��ϱ������õ�ijЩ���ɣ����Ը�����ó��Ĺ����Ʋ�ClԪ�ص�ԭ������n����ֵ�� ����д���������� ��
(4)ԭ������Ϊ9��Ԫ�ص�ԭ�ӵõ����Ӻ��γ�-1�۵����ӣ������ӷ����� ��
(5)���ñ��е�Ԫ�ط���д������Ҫ��Ļ�ѧ��Ӧ����ʽ��һ�����н����μӵĻ��Ϸ�Ӧ�� �����ַǽ������ʷ����Ļ��Ϸ�Ӧ�� ��
��1���裬7
��2��13��Al
��3��17��ÿһ���д����ң�ԭ���������ε���
��4��F-
��5��2Mg + O2 2MgO ��C + O2
2MgO ��C + O2 CO2
CO2
��2��13��Al
��3��17��ÿһ���д����ң�ԭ���������ε���
��4��F-
��5��2Mg + O2
 2MgO ��C + O2
2MgO ��C + O2 CO2
CO2 
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ




