��Ŀ����
����Ŀ������ͼ��ʵ�������������Լ�ƿ�ϱ�ǩ�IJ������ݣ�����ϸ�Ķ������������Ϣ���㣺
��1��������������������5����ϡ������Һ100g����Ҫ������������Ϊ37����Ũ������ٺ���?����������ȷ��0.1 mL��
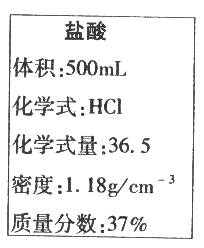
��2����ѧ��ȤС���ͬѧ����һ��ֽ���ŷŵ���ˮ���м�⣬��������Ҫ��Ⱦ��Ϊ�������ơ�Ϊ�˲ⶨ��ˮ���������Ƶ�����������ȡ50g��ˮ���ձ��У���μ���������������Ϊ5����ϡ������36.5gʱǡ����ȫ��Ӧ����ˮ�е������ɷֲ������ᷴӦ��������ˮ���������Ƶ���������?����������ȷ��1������
���𰸡�(1)11.5ml(2) 4%
��������
�������������Ҫ��������Ϊ37����Ũ��������Ϊx
100g��5��=x��1.18g/cm-3��37��
x=11.5cm-3=11.5mL
��2����50g��ˮ���������Ƶ�����Ϊy
NaOH+HCl=NaCl+H2O
40 36.5
x 36.5g��5��
�б���ʽ�ã�40��X=36.5����36.5��*5%����� x=2g
����ˮ���������Ƶ���������Ϊ2��/50����100��=4��

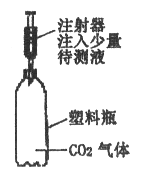
����Ŀ��ʵ��������ƿ��ǩ�������ɫ��Һ�ס��ң�ֻ֪�����Ƿֱ���ϡ���������������Һ����ͬѧ��Ʋ�ͬ�����������ǡ���Ҫ��ش��������⣺
��1���ڲ�ѡ�����ָʾ����pH��ֽ������£�ѡ��ͬ�������ʽ������֡�
�����Լ� | ʵ������ͽ��� | ������Ӧ�Ļ�ѧ����ʽ | |
����һ | п�� | �����ݲ�������ϡ���ᣬ�����ݲ�����������������Һ | Zn+2HCl=ZnCl2+H2�� |
������ |
��2��ͬѧ���ڽ���ʵ�鷽��ʱ��С����Ƶġ��ö�����̼�������Լ���������һ�����ۡ������۵㣺�������𣻷����۵㣺������С����ͬ�����Ĺ۵㣬��������_______________��С������ͬ�����Ĺ۵㣬�����Ҳ��ͬ�Ļ�������ο���ͼװ��˵����֤�ķ�������Ҫд�������������ۣ���__________________��