��Ŀ����
2010���Ϻ������Ἢ���������������̬��ˮ��ˮ��������ԴȪ�����˽���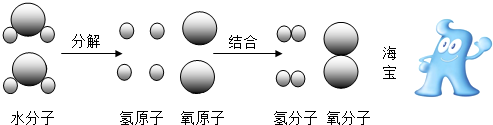
��1��ˮ���� ��ɵģ�ˮ��������Ԫ�ص�������Ϊ ���ϼ��� ��
��2����ͼ�ǵ��ˮ������ˮ���ӷֽ����ʾ��ͼ
����ͼ�е���Ϣ����֪������ѧ�仯�� �Ĺ��̣��ڵ��ˮ�Ĺ����в�������� ������ţ�
����ԭ�Ӣ���ԭ�Ӣ�ˮ����
��3�����������������ʣ���Ҫ���д��һ����ˮ�йصĻ�ѧ����ʽ
��ѡ���ʶ�����̼�������ơ����������顢����
����ˮ�μӵĻ��Ϸ�Ӧ
����ˮ�����Ļ�ѧ��Ӧ
��4����Ȼ���ˮ�к��ж������ʣ�ͨ����Ӳˮ������ �����������ƣ��������ˮ������ �ķ�����ȥˮ�еIJ���������
��5��������������ȱˮ����������ӷ�ֹˮ��Ⱦ����Լ��ˮ�ȷ���д��һ���Լ������ܼ��ľ������� ��

��1��ˮ����
��2����ͼ�ǵ��ˮ������ˮ���ӷֽ����ʾ��ͼ
����ͼ�е���Ϣ����֪������ѧ�仯��
����ԭ�Ӣ���ԭ�Ӣ�ˮ����
��3�����������������ʣ���Ҫ���д��һ����ˮ�йصĻ�ѧ����ʽ
��ѡ���ʶ�����̼�������ơ����������顢����
����ˮ�μӵĻ��Ϸ�Ӧ
����ˮ�����Ļ�ѧ��Ӧ
��4����Ȼ���ˮ�к��ж������ʣ�ͨ����Ӳˮ������
��5��������������ȱˮ����������ӷ�ֹˮ��Ⱦ����Լ��ˮ�ȷ���д��һ���Լ������ܼ��ľ�������
��������1������ˮ����ɷ��������ݻ������и�Ԫ��������=��ԭ�ӵ����ԭ��������ԭ�Ӹ���֮�ȣ����ݳ���Ԫ�صĻ��ϼ۽��з������
��2�����ݶԵ��ˮ����ʾ��ͼ�����⣬�����ڴ˱仯�����пɵõ��Ĺ��ɻ���ʶ��
��3�����ݸ����ʵ�������д��ѧ����ʽ��
��4��Ӳˮ����ˮ���������ڸ�þ���ӵĶ��٣������÷���ˮ�����ù��˵ķ�����ȥˮ�еIJ��������ʣ�
��5�����ݱ���ˮ��Դ���������ճ�����ʵ��������з�������⣮
��2�����ݶԵ��ˮ����ʾ��ͼ�����⣬�����ڴ˱仯�����пɵõ��Ĺ��ɻ���ʶ��
��3�����ݸ����ʵ�������д��ѧ����ʽ��
��4��Ӳˮ����ˮ���������ڸ�þ���ӵĶ��٣������÷���ˮ�����ù��˵ķ�����ȥˮ�еIJ��������ʣ�
��5�����ݱ���ˮ��Դ���������ճ�����ʵ��������з�������⣮
����⣺��1��ˮ��������Ԫ����ɵģ�ˮ���⡢��Ԫ�ص�������Ϊ��1��2������16��1��=1��8��ˮ������+1�ۣ�����-2�ۣ�
�ʴ�Ϊ����Ԫ�غ���Ԫ�أ�1��8���⣺+1������-2��
��2������ͼ�е���Ϣ����֪������ѧ�仯�Ƿ��ӷ��ѳ�ԭ�ӣ�ԭ��������ϳ��µķ��ӵĹ��̣��ڵ��ˮ�Ĺ����в����������ԭ�Ӻ���ԭ�ӣ�
�ʴ�Ϊ�����ӷ��ѳ�ԭ�ӣ�ԭ��������ϳ��µķ��ӣ��٢ڣ�
��3���ٶ�����̼��ˮ��Ӧ����̼�ᡢ�����ƺ�ˮ��Ӧ�����������ƶ����ڻ��Ϸ�Ӧ��
������ȼ�ա�����ȼ�շ�Ӧ���ж���ˮ���ɣ�
�ʴ�Ϊ��CaO+H2O=Ca��OH��2��2H2+O2
2H2O��
��4�����÷���ˮ�������ˮ�����ù��˵ķ�����ȥˮ�еIJ��������ʣ�
�ʴ�Ϊ������ˮ�����ˣ�
��5�����ݱ���ˮ��Դ���������ճ�����ʵ���������ֹˮ��Ⱦ����ͽ�Լ��ˮ��������������������������������ʹ�ý�ˮ��ͷ���ú�š��ˮ��ͷ�ȣ�
�ʴ�Ϊ�������������������ʹ�ý�ˮ��ͷ���ú�š��ˮ��ͷ�ȣ�
�ʴ�Ϊ����Ԫ�غ���Ԫ�أ�1��8���⣺+1������-2��
��2������ͼ�е���Ϣ����֪������ѧ�仯�Ƿ��ӷ��ѳ�ԭ�ӣ�ԭ��������ϳ��µķ��ӵĹ��̣��ڵ��ˮ�Ĺ����в����������ԭ�Ӻ���ԭ�ӣ�
�ʴ�Ϊ�����ӷ��ѳ�ԭ�ӣ�ԭ��������ϳ��µķ��ӣ��٢ڣ�
��3���ٶ�����̼��ˮ��Ӧ����̼�ᡢ�����ƺ�ˮ��Ӧ�����������ƶ����ڻ��Ϸ�Ӧ��
������ȼ�ա�����ȼ�շ�Ӧ���ж���ˮ���ɣ�
�ʴ�Ϊ��CaO+H2O=Ca��OH��2��2H2+O2
| ||
��4�����÷���ˮ�������ˮ�����ù��˵ķ�����ȥˮ�еIJ��������ʣ�
�ʴ�Ϊ������ˮ�����ˣ�
��5�����ݱ���ˮ��Դ���������ճ�����ʵ���������ֹˮ��Ⱦ����ͽ�Լ��ˮ��������������������������������ʹ�ý�ˮ��ͷ���ú�š��ˮ��ͷ�ȣ�
�ʴ�Ϊ�������������������ʹ�ý�ˮ��ͷ���ú�š��ˮ��ͷ�ȣ�
�����������ѶȲ�����ͬѧ��������û�ѧʽ���йؼ�����з������⡢�����������������ݱ���ˮ��Դ���������ճ�����ʵ��������з�������������ȣ�

��ϰ��ϵ�д�
�����Ŀ
 2��2010���Ϻ��������й���--������֮�ڡ�����ͼ��������ṹ���ù���Q460���Ƴɵģ������й�Q460�ֵ������У����ڻ�ѧ���ʵ��ǣ�������
2��2010���Ϻ��������й���--������֮�ڡ�����ͼ��������ṹ���ù���Q460���Ƴɵģ������й�Q460�ֵ������У����ڻ�ѧ���ʵ��ǣ�������