��Ŀ����
 ˮ��������ԴȪ��Ҳ�Dz���ȱ�ٵ���Դ��
ˮ��������ԴȪ��Ҳ�Dz���ȱ�ٵ���Դ��
��1��ij��Ȫˮ����Ҫ�����ʳɷּ��������±���
| �ɷ� | Ca | K | Zn | F |
| ������mg/L�� | 20 | 3 | 0.06 | 0.02 |
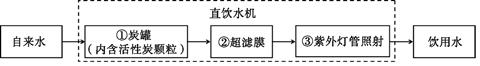
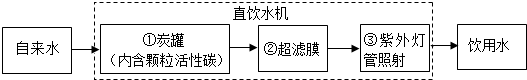
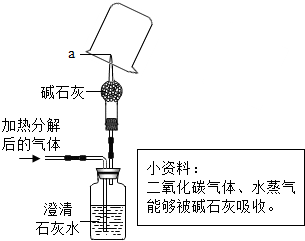
��2�����������ܡ�����ͼ����һ�ֿ�������Я����С��ˮ������������װ����˿��ע���˻���̿�͵����֬�ȣ����л���̿��________���˵����ã��˾�ˮ���������ˮ��________����������������
��3���������������ˮ����Ⱦ����________������ĸ����
A����ҵ��ˮֱ���ŷš��������� B����ҵ�����������ŷ�
C����ֹʹ�ú���ϴ�·ۡ������� D������ʹ�û��ʡ�ũҩ
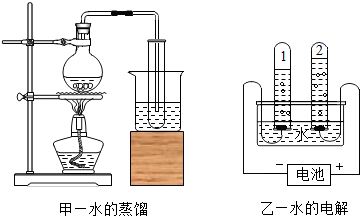
��4����ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ����________������ĸ����
A��������������ȼ������ˮ���� B��ˮ������
C��ˮ�ĵ�⡡���������������� D��ˮ�ľ�����
�⣺��1��ˮ����Ҫ�������еġ�Ca��K��Zn��F���Ȳ����Ե��ʡ����ӡ�ԭ�ӵ���ʽ���ڣ�����ָԪ�أ�
�ʴ�Ϊ��Ԫ�أ�
��2������̿�����ɶ�Ľṹ���к�ǿ�������ԣ���ˮʱ�� �������˵����ã���ˮ���������ˮ���Ժ��п����Եijɷ֣������ڻ���
�ʴ�Ϊ������������
��3��A������ҵ��ˮֱ���ŷŻ����ˮ��Ⱦ����A�������⣻
B����ҵ�����������ŷŲ������ˮ��Ⱦ����B���������⣻
C����ֹʹ�ú���ϴ�·۲�����Ⱦˮ��Դ����C���������⣻
D������ʹ��ũҩ�����ʻ���Ⱦˮ�壬��D�������⣮
��ѡAD��
��4����ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ��Ҫ���������غ㶨�ɣ���Ӧǰ��Ԫ������䣬������������ȼ������ˮ��ˮͨ����������������������˵��ˮ�к�����Ԫ�غ���Ԫ�أ�
��ѡAC��
��������1��ˮ����Ҫ�������еġ�Ca��K��Zn��F���Ȳ����Ե��ʡ����ӡ�ԭ�ӵ���ʽ���ڣ�����ָԪ�أ�ͨ����Ԫ�ؼ�����ռ������������������������
��2�����ݻ���̿�����ú;������ˮ�ijɷֻش�
��3������ˮ��Ⱦ��֪ʶ���з�����
��4����ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ��Ҫ���������غ㶨�������ǣ�
������������Ҫ������Ԫ�ء����ʵķ��ࡢˮ��Ⱦ�Լ�ˮ����ɵȻ�ѧ֪ʶ���ѶȲ���
�ʴ�Ϊ��Ԫ�أ�
��2������̿�����ɶ�Ľṹ���к�ǿ�������ԣ���ˮʱ�� �������˵����ã���ˮ���������ˮ���Ժ��п����Եijɷ֣������ڻ���
�ʴ�Ϊ������������
��3��A������ҵ��ˮֱ���ŷŻ����ˮ��Ⱦ����A�������⣻
B����ҵ�����������ŷŲ������ˮ��Ⱦ����B���������⣻
C����ֹʹ�ú���ϴ�·۲�����Ⱦˮ��Դ����C���������⣻
D������ʹ��ũҩ�����ʻ���Ⱦˮ�壬��D�������⣮
��ѡAD��
��4����ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ��Ҫ���������غ㶨�ɣ���Ӧǰ��Ԫ������䣬������������ȼ������ˮ��ˮͨ����������������������˵��ˮ�к�����Ԫ�غ���Ԫ�أ�
��ѡAC��
��������1��ˮ����Ҫ�������еġ�Ca��K��Zn��F���Ȳ����Ե��ʡ����ӡ�ԭ�ӵ���ʽ���ڣ�����ָԪ�أ�ͨ����Ԫ�ؼ�����ռ������������������������
��2�����ݻ���̿�����ú;������ˮ�ijɷֻش�
��3������ˮ��Ⱦ��֪ʶ���з�����
��4����ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ��Ҫ���������غ㶨�������ǣ�
������������Ҫ������Ԫ�ء����ʵķ��ࡢˮ��Ⱦ�Լ�ˮ����ɵȻ�ѧ֪ʶ���ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
ˮ��������ԴȪ��Ҳ�Dz���ȱ�ٵ���Դ��
��1��ij��Ȫˮ����Ҫ�����ʳɷּ��������±���
| �ɷ� | Ca | K | Zn | F |
| ������mg/L�� | 20 | 3 | 0.06 | 0.02 |
��2��ˮ��Ⱦ�������أ�ˮ��Դ�ı����ͺ����������ܵ����ǵ��ձ��ע����������������й����⣺
��������ˮ������ˮ����ˮ�����ڴ�������� ��
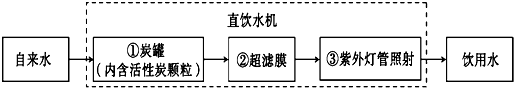
����ijѧУ��ˮ�����Խ�����ˮ����Ϊ����ˮ�����д�����������ͼ��ʾ��
�ٶ�Ӧ�������� ������ĸ��ţ���ͬ�����۶�Ӧ�������� ��
A��ɱ������ B���������� C���������� D������
�����������������ˮ����Ⱦ���� ��
A������ʹ�û��ʡ�ũҩ B����ҵ�����������ŷ� C����ֹʹ�ú���ϴ�·�
��������ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ���� ��
����ˮ������ �£�ˮ�ĵ�� �ã�ˮ�ľ���

 ˮ��������ԴȪ��Ҳ�Dz���ȱ�ٵ���Դ��
ˮ��������ԴȪ��Ҳ�Dz���ȱ�ٵ���Դ��