��Ŀ����
ʵ���Ҳ���������װ����ͼ��ʾ����ش��������⣺
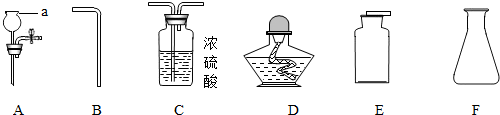
��1��д����ͼ������a�����ƣ�______��
��2����ʵ��������һ�ֹ����һ��Һ���ϣ���ȡ���ռ�һƿ����ĵ��ʻ������壬��ѡ���������������ղ����������������ҵ�˳�����ӳ�һ��ʵ��װ�ã����д��ĸ��ţ���______��______��______��______��______��
���Ʊ��������壬��Ӧ�Ļ�ѧ����ʽ��______��
���Ʊ����������壬��Ӧ�Ļ�ѧ����ʽ��______��
��3��ʵ���У����Ӳ����ܺͽ�Ƥ�ܵķ�����______��
�⣺��1��a�dz���©����
��2���������ǹ�Һ��Ӧ����ķ���װ�ã���ҪA��Fװ�ã�Ũ���������ˮ�ԣ���������������ռ�������Ҫ�����ܺͼ���ƿ�������ӵ�˳���ǣ�F��A��C��B��E��
����ȡ�������ǵ�����������Ӧ�ķ���ʽΪ��2H2O2 2H2O+O2����
2H2O+O2����
����ȡ�������ǻ����������̼����Ӧ�ķ���ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
��3�����Ӳ����ܺͽ�Ƥ�ܵķ����ش��ȰѲ����ܿ���ˮʪ��Ȼ�������������ɰѲ����ܲ��뽺Ƥ�ܣ�
�ʴ�Ϊ����1������©����
��2��F A C B E�� 2H2O2 2H2O+O2����CaCO3+2HCl=CaCl2+H2O+CO2����
2H2O+O2����CaCO3+2HCl=CaCl2+H2O+CO2����
��3���ȰѲ����ܿ���ˮʪ��Ȼ�������������ɰѲ����ܲ��뽺Ƥ�ܣ�
��������1���������ճ����Ļ�ѧ�������ƺ���;��
��2�����ݷ���װ�õ����ʡ�����ĸ���ռ�����������ѡ������ӣ�
��Һ��ȡ��������dz����������������̼����ȡ��д����Ӧ�ķ���ʽ��
��3���������Ӳ����ܺͽ�Ƥ�ܵķ����ش��ȰѲ����ܿ���ˮʪ����Ħ������Ȼ�������������ɰѲ����ܲ��뽺Ƥ�ܣ�
���������⿼����ʵ���ҳ����������ȡԭ������д������װ�á��ռ�װ�õ�ѡ���Լ��������ȣ��ۺ��ԱȽ�ǿ���ؼ�����ȷ����װ�á��ռ�����ѡ������ݼ���ص�֪ʶ���ܹ�����ѧ����֪ʶǨ��������������Ҫ�ص����յ�֪ʶ��Ҳ���п����������ͣ�
��2���������ǹ�Һ��Ӧ����ķ���װ�ã���ҪA��Fװ�ã�Ũ���������ˮ�ԣ���������������ռ�������Ҫ�����ܺͼ���ƿ�������ӵ�˳���ǣ�F��A��C��B��E��
����ȡ�������ǵ�����������Ӧ�ķ���ʽΪ��2H2O2
 2H2O+O2����
2H2O+O2��������ȡ�������ǻ����������̼����Ӧ�ķ���ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
��3�����Ӳ����ܺͽ�Ƥ�ܵķ����ش��ȰѲ����ܿ���ˮʪ��Ȼ�������������ɰѲ����ܲ��뽺Ƥ�ܣ�
�ʴ�Ϊ����1������©����
��2��F A C B E�� 2H2O2
 2H2O+O2����CaCO3+2HCl=CaCl2+H2O+CO2����
2H2O+O2����CaCO3+2HCl=CaCl2+H2O+CO2������3���ȰѲ����ܿ���ˮʪ��Ȼ�������������ɰѲ����ܲ��뽺Ƥ�ܣ�
��������1���������ճ����Ļ�ѧ�������ƺ���;��
��2�����ݷ���װ�õ����ʡ�����ĸ���ռ�����������ѡ������ӣ�
��Һ��ȡ��������dz����������������̼����ȡ��д����Ӧ�ķ���ʽ��
��3���������Ӳ����ܺͽ�Ƥ�ܵķ����ش��ȰѲ����ܿ���ˮʪ����Ħ������Ȼ�������������ɰѲ����ܲ��뽺Ƥ�ܣ�
���������⿼����ʵ���ҳ����������ȡԭ������д������װ�á��ռ�װ�õ�ѡ���Լ��������ȣ��ۺ��ԱȽ�ǿ���ؼ�����ȷ����װ�á��ռ�����ѡ������ݼ���ص�֪ʶ���ܹ�����ѧ����֪ʶǨ��������������Ҫ�ص����յ�֪ʶ��Ҳ���п����������ͣ�

��ϰ��ϵ�д�
�����Ŀ

 ʽΪ
ʽΪ


