��Ŀ����
��ij���������֪�û�����п��ܺ���FeCl3��NaCl��NH4NO3��CuSO4���������е����ֻ���֡�Ϊ̽������ɣ���������ʵ�飨����������з����ķ�Ӧ��ǡ����ȫ��Ӧ����
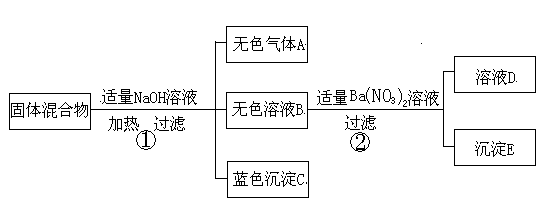
�Ը���ʵ����̺ͷ�����������д���¿հף�
��1����ʪ��ĺ�ɫʯ����ֽ��������A����ֽ��__________ɫ��C�Ļ�ѧʽΪ�� ��
��2������������������������У��϶������ڵ�������______________��д��ѧʽ����
��3������ҺD�У�һ�����е��������ǣ�д���ӷ��ţ�_____________��
��4��������з�����Ӧ�Ļ�ѧ����ʽΪ______________________________________��
��5������������������������У�������ȷ�����ڵ������ǣ�д��ѧʽ��________��
Ҫ��һ��ȷ���ù�������ɷ֣�������ҺD��ʵ�飬���Ҫ˵��ʵ��������衢������������ ��

�Ը���ʵ����̺ͷ�����������д���¿հף�
��1����ʪ��ĺ�ɫʯ����ֽ��������A����ֽ��__________ɫ��C�Ļ�ѧʽΪ�� ��
��2������������������������У��϶������ڵ�������______________��д��ѧʽ����
��3������ҺD�У�һ�����е��������ǣ�д���ӷ��ţ�_____________��
��4��������з�����Ӧ�Ļ�ѧ����ʽΪ______________________________________��
��5������������������������У�������ȷ�����ڵ������ǣ�д��ѧʽ��________��
Ҫ��һ��ȷ���ù�������ɷ֣�������ҺD��ʵ�飬���Ҫ˵��ʵ��������衢������������ ��
�� Cu(OH)2 FeCl3 NO3- Na2SO4+Ba(NO3)2=BaSO4��+2NaNO3
NaCl ����ҺD�м�����������Һ�����ְ׳������μ�ϡ����������ܽ⣬˵��ԭ������к���NaCl���������ְ�ɫ��������ԭ������в�����NaCl��
NaCl ����ҺD�м�����������Һ�����ְ׳������μ�ϡ����������ܽ⣬˵��ԭ������к���NaCl���������ְ�ɫ��������ԭ������в�����NaCl��
�����������1�����ݻ����Ŀ�����ɷ��������������������ƹ��ȣ���Ӧ������ɫ���������ֻ������泥��Ҷ��߷�Ӧ���ɰ�������������ˮ�ʼ��ԣ���ʹʪ��ĺ�ɫʯ����ֽ�����������Ŀ�������У�ֻ������ͭ��������������Һ��Ӧ��������ɫ������������ͭ����
��2�������Ȼ�����������������Һ��Ӧ���ɺ��ɫ�����������������������������֪����Ӧ���������з����ķ�Ӧ��ǡ����ȫ��Ӧ���Ҳ������ֻ�õ���ɫ�����������ɫ����������˵��һ��û���Ȼ�����
��3��������������������Ʒ�Ӧ���������ơ�ˮ�Ͱ���������ͭ���������Ʒ�Ӧ����������ͭ�����������ƣ�������ҺB��һ�����������ƺ������ƣ����������������������ᱵ��Ӧ���������ƺ����ᱵ�������������������ᱵ����Ӧ��������ҺD�У��϶����ڵ�����������������ӡ�
��4����������ķ�����������У������������������ᱵ��Ӧ���������ƺ����ᱵ�������ʷ�����Ӧ�Ļ�ѧ����ʽΪNa2SO4+Ba(NO3)2=BaSO4��+2NaNO3��
��5���������Ϸ������������������У�������ȷ�����ڵ�������NaCl��Ҫ��һ��ȷ���ù�������ɷ֣�������ҺD�м�����������Һ�������ְ׳������μ�ϡ����������ܽ⣬˵��ԭ������к���NaCl���������ְ�ɫ��������ԭ������в�����NaCl��
�������������⣬��Ҫ�������ʼ�ķ�Ӧ���ɡ�ץס��Ӧ�������ֵ�����Ѱ��ͻ�ƿڣ������ʵ����Ի�Ӧʱ����������ȣ������ƶϼ��ɣ�����Ҫ��ѧ����ƽʱ��ѧϰ��Ҫע������ʵ����Ի�Ӧʱ�����������֪ʶ���д�����

��ϰ��ϵ�д�
�����Ŀ