��Ŀ����
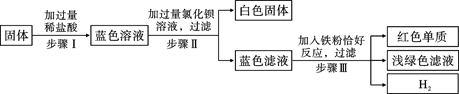
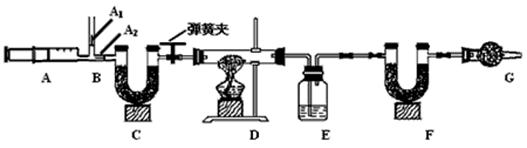
ijƷ�ƴ����к��������Ȼ��ơ�ij��ѧ̽��С��Ϊ�˲ⶨ��Ʒ�ƴ���Ĵ��ȣ���̼���Ƶ�����������������һͬѧ�����ͼ��ʾʵ�飺
(1)Aװ���з�Ӧ�Ļ�ѧ����ʽΪ ��
(2)Bװ�õ������� ��Cװ���з�Ӧ�Ļ�ѧ����ʽΪ ��
(3) Dװ�õ������� ��
(4)С������ܷ�������������ͬѧ�Ǿ���������Ϊ���ܣ������� ��
(5)С�������Ҫʹ�ⶨ�����ȷ��Ӧ��Aװ�øij�ͼ15��ʾװ�ã����ڷ�Ӧǰ�����������Ŀ����_____________________;��Ӧ��Ҫ�����������Ŀ����_____________________.
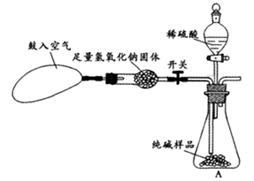
(6)�Ƶô�����Ʒ������Ϊ12.0 g��ʵ��ǰ����Cװ�ã�����ҩƷ���������ֱ�Ϊ61.2 g��65.6 g����ô�����Ʒ�Ĵ���Ϊ %����ȷ��0.1%����

(1)Aװ���з�Ӧ�Ļ�ѧ����ʽΪ ��
(2)Bװ�õ������� ��Cװ���з�Ӧ�Ļ�ѧ����ʽΪ ��
(3) Dװ�õ������� ��
(4)С������ܷ�������������ͬѧ�Ǿ���������Ϊ���ܣ������� ��
(5)С�������Ҫʹ�ⶨ�����ȷ��Ӧ��Aװ�øij�ͼ15��ʾװ�ã����ڷ�Ӧǰ�����������Ŀ����_____________________;��Ӧ��Ҫ�����������Ŀ����_____________________.

(6)�Ƶô�����Ʒ������Ϊ12.0 g��ʵ��ǰ����Cװ�ã�����ҩƷ���������ֱ�Ϊ61.2 g��65.6 g����ô�����Ʒ�Ĵ���Ϊ %����ȷ��0.1%����
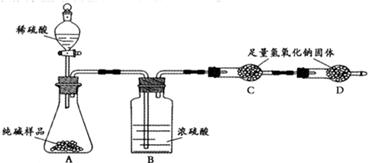
��1��Na2CO3+H2SO4��Na2SO4+H2O+CO2��
��2����ȥˮ���� 2NaOH + CO2 = Na2CO3+ H2O
��3����ֹ�����ж�����̼��ˮ��������Cװ��
(4)����ӷ����Ȼ������屻װ��C���գ�Ӱ��ʵ��ⶨ
(5)�ų�װ���ж�����̼ ʹ���ɵĶ�����̼ȫ������Cװ��
(6) 88.3
��2����ȥˮ���� 2NaOH + CO2 = Na2CO3+ H2O
��3����ֹ�����ж�����̼��ˮ��������Cװ��
(4)����ӷ����Ȼ������屻װ��C���գ�Ӱ��ʵ��ⶨ
(5)�ų�װ���ж�����̼ ʹ���ɵĶ�����̼ȫ������Cװ��
(6) 88.3
���������(1)Aװ����Ϊϡ�����̼���Ƶķ�Ӧ�����������ơ�ˮ�Ͷ�����̼����2��Cװ����ʢ����Ũ���ᣬĿ��������ˮ������Cװ�����������Ƶ����������ն�����̼��(3)Dװ�õ����������Ƶ����������տ����е�ˮ�����Ͷ�����̼����ֹ����װ�������(4)������ϡ���ᣬ����ӷ����Ȼ������屻װ��C���գ�Ӱ��ʵ��ⶨ��(5)Aװ�õ�ȱ���Dz����Ķ�����̼����һ���ֲ�����Aװ���У����Է�Ӧǰ���������Ϊ���ų�װ���ж�����̼����Ӧ����������Ϊ��ʹ���ɵĶ�����̼ȫ������Cװ�ã�
(6)�贿����Ʒ��̼���Ƶ�����Ϊ
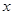 ��
��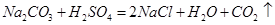
106 44
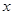 65.6g-61.2g
65.6g-61.2g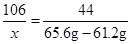 �����
�����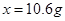
��ô�����Ʒ�Ĵ���Ϊ88.3%.
����������һ���dz����͵�̽���⣬��Ŀ����֪ʶ��û��ѧ������Ϊİ���������ص㿼��ķ�Ӧ��˼�룬װ�õȣ�����������Ŀ��Ҫ���£���ϸ���⼴�ɡ�

��ϰ��ϵ�д�
�����Ŀ
 Na2CO3��+CO2 ��+H2O ��
Na2CO3��+CO2 ��+H2O ��