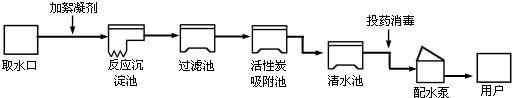
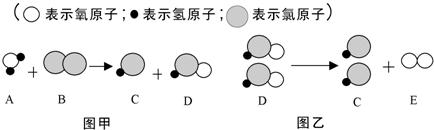
��Ŀ����
���ϼ۵ı�ʾ������һ����ɣ�
��1��
��Mg2+��ʲô��ͬ��
��2�����ϼ�������������Ԫ��ͨ����
��1��
| +2 | Mg |
ǰ�߱�ʾþԪ�صĻ��ϼ�Ϊ+2�ۣ����߱�ʾ1��þ���Ӵ�2����λ�����
ǰ�߱�ʾþԪ�صĻ��ϼ�Ϊ+2�ۣ����߱�ʾ1��þ���Ӵ�2����λ�����
����2�����ϼ�������������Ԫ��ͨ����
-2
-2
�ۣ�����Ԫ��ͨ����+1
+1
�ۣ��۽���Ԫ�ظ��ǽ���Ԫ�ػ���ʱ������Ԫ������
��
�ۣ��ǽ���Ԫ������
��
�ۣ���һЩԪ���ڲ�ͬ�Ļ������п��Բ�ͬ�Ļ��ϼۣ��ݶ����κε��ʣ���Ԫ�صĻ��ϼ�Ϊ��
��
�����ڻ������У�Ԫ���������ϼ۵Ĵ�����Ϊ��
��
����������1�����ݻ��ϼۺ����ӵIJ�ͬ�㿼�ǣ���2������Ԫ�ػ��ϼ۵�һ����ɷ�����
����⣺��1��Ԫ�صĻ��ϼ�д�ڷ��ŵ����Ϸ�����д�����ţ���д��ֵ�������������д�ڷ��ŵ����Ͻǣ���д��ֵ����д�����ţ�
��2����Ԫ��ͨ����-2�ۣ���Ԫ��ͨ����+1�ۣ�����Ԫ������������һ������4������ʧȥ���ӣ������ۣ��ǽ���Ԫ������������һ����ڻ����4�����õ��ӣ�����һ���Ը��ۣ����ʻ��ϼ�Ϊ�㣬�ڻ��������������ϼ۴�����Ϊ�㣮
�ʴ�Ϊ����1��ǰ�߱�ʾþԪ�صĻ��ϼ�Ϊ+2�ۣ����߱�ʾ1��þ���Ӵ�2����λ����ɣ���2��-2��+1�����������㣻�㣮
��2����Ԫ��ͨ����-2�ۣ���Ԫ��ͨ����+1�ۣ�����Ԫ������������һ������4������ʧȥ���ӣ������ۣ��ǽ���Ԫ������������һ����ڻ����4�����õ��ӣ�����һ���Ը��ۣ����ʻ��ϼ�Ϊ�㣬�ڻ��������������ϼ۴�����Ϊ�㣮
�ʴ�Ϊ����1��ǰ�߱�ʾþԪ�صĻ��ϼ�Ϊ+2�ۣ����߱�ʾ1��þ���Ӵ�2����λ����ɣ���2��-2��+1�����������㣻�㣮
�����������ؼ���Ҫ֪�����ϼ۵ı귨�����ӵ�д������Ϥ���ϼ۵�һ����ɣ�����������ý��ʵ�����⣮

��ϰ��ϵ�д�
�����Ŀ


 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�