��Ŀ����
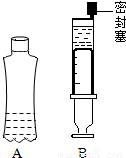 ��ѧ���ϣ���ʦ��CO2�ֱ�ͨ�����ʯ��ˮ������������Һ�У����ǹ۲쵽ǰ�߱�룬����û����������CO2�� NaOH�Ƿ����˻�ѧ��Ӧ�أ��ס�����ͬѧ�������ͼ��ʾ��A��B����ʵ������֤���۲쵽�����ǣ�װ��A�����ϱ�⣬װ��B���������˶���
��ѧ���ϣ���ʦ��CO2�ֱ�ͨ�����ʯ��ˮ������������Һ�У����ǹ۲쵽ǰ�߱�룬����û����������CO2�� NaOH�Ƿ����˻�ѧ��Ӧ�أ��ס�����ͬѧ�������ͼ��ʾ��A��B����ʵ������֤���۲쵽�����ǣ�װ��A�����ϱ�⣬װ��B���������˶�����1����ͬѧ��Ϊ������ʵ�鶼���У��䷴Ӧԭ���������û�ѧ����ʽ��ʾΪ______��
��2����ͬѧ��������ɣ�����Ϊ������ʵ�鶼����֤��ʹ������ѹǿ��С��ԭ����CO2��NaOH��Ӧ������CO2����ˮ����ͬѧ��Ϊ���Բ���һ��ʵ�����ش�����⣬��ʵ����______����ֻ����Aװ��--��Ȫˮƿ���У�
���𰸡���������һ�ʸ����������Ƶ����ʿ�д��������ѹǿ��С��ԭ�ɶ�Ƕ����ʵ��֤����Ȫˮƿ����ԭ��
����⣺��1����CO2����NaOH��Һ������̼���ƺ�ˮ��CO2+2NaOH=Na2CO3+H2O��
��2��������Ĺؼ��ǵ�CO2��NaOH��Һ��Ӧʱ��Ҳ����ͬʱ����ˮ�����Ҷ����ܵ��¿�Ȫˮƿ������Է�������������һ�����Ա����飬ȡ����ͬ����Ŀ�Ȫˮƿ����CO2��ע��ͬ�����ˮ��NaOH��Һ����ƿ�����Ƚ�����Ȫˮƿ���ij̶ȣ����������Ǵ��Ŀ�Ȫˮƿ�ǣ������������ᣬ��������ƿ�ǣ�š��������Ȫˮƿ���¹�������֤����CO2������������Һ�����˷�Ӧ��
�����1��CO2+2NaOH=Na2CO3+H2O��
��2�������Ա����飬ȡ����ͬ����Ŀ�Ȫˮƿ����CO2��ע��ͬ�����ˮ��NaOH��Һ����ƿ�����Ƚ�����Ȫˮƿ���ij̶ȣ������ߴ��Ŀ�Ȫˮƿ�ǣ������������ᣬ��������ƿ�ǣ�š��������Ȫˮƿ���¹�������֤����CO2������������Һ�����˷�Ӧ����
������������Ҫ������ѧ����ʵ����۵ķ���̽�����������������ѧ����������ͽ�������������
����⣺��1����CO2����NaOH��Һ������̼���ƺ�ˮ��CO2+2NaOH=Na2CO3+H2O��
��2��������Ĺؼ��ǵ�CO2��NaOH��Һ��Ӧʱ��Ҳ����ͬʱ����ˮ�����Ҷ����ܵ��¿�Ȫˮƿ������Է�������������һ�����Ա����飬ȡ����ͬ����Ŀ�Ȫˮƿ����CO2��ע��ͬ�����ˮ��NaOH��Һ����ƿ�����Ƚ�����Ȫˮƿ���ij̶ȣ����������Ǵ��Ŀ�Ȫˮƿ�ǣ������������ᣬ��������ƿ�ǣ�š��������Ȫˮƿ���¹�������֤����CO2������������Һ�����˷�Ӧ��
�����1��CO2+2NaOH=Na2CO3+H2O��
��2�������Ա����飬ȡ����ͬ����Ŀ�Ȫˮƿ����CO2��ע��ͬ�����ˮ��NaOH��Һ����ƿ�����Ƚ�����Ȫˮƿ���ij̶ȣ������ߴ��Ŀ�Ȫˮƿ�ǣ������������ᣬ��������ƿ�ǣ�š��������Ȫˮƿ���¹�������֤����CO2������������Һ�����˷�Ӧ����
������������Ҫ������ѧ����ʵ����۵ķ���̽�����������������ѧ����������ͽ�������������

��ϰ��ϵ�д�
 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
�����Ŀ