��Ŀ����
��֪X���ж������壬����������ѪҺ�е�Ѫ�쵰��ϣ������Dz�����ˮ�����壬Y�Dz�֧��ȼ�յ����壬Z�Dz�����ˮ�Ĺ��壬X��Y��Z֮��������ת����ϵ����ش��������⡣
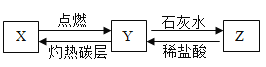
(1)д��X�Ļ�ѧʽ______��
(2)д��Z��ϡ���ᷴӦ����Y�Ļ�ѧ����ʽ��___��
(3)�����ʵ������ṹ�������ʵ����ʡ�����Ҫ�Ļ�ѧ˼�롣����X��Y��������ͬ��Ԫ�أ������ǵ��������ʡ���ѧ���ʶ���ͬ����ٳ�X��Y���ʲ�ͬ��һ������_____��
 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�������;�㷺����ṹ�����ʵ��ǻ�ѧ����Ҫ�о����ݡ�
��1����ͼ������ԭ�ӽṹʾ��ͼ������˵������ȷ����_____��
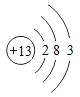
A ��ԭ�ӵ�������Ϊ13 B �ڻ���������ͨ����+3��
C ���ǵؿ��к�������Ԫ�� D �������������������������õĵ�����
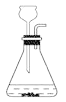
��2��ij��ѧС����һ����AgNO3��Cu(NO3)2�����Һ��������ͼʵ�飬������ҺA����B�ijɷֽ����˷�����ʵ��̽����
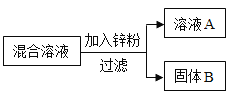
��������⣩��ҺA�е����ʿ�������Щ��
���������룩��ֻ��Zn(NO3)2��Zn(NO3)2��AgNO3��Zn(NO3)2��Cu(NO3)2��Zn(NO3)2��AgNO3��Cu(NO3)2
���������ۣ��������IJ�����_____�����ţ�����������_____��
��ʵ��̽����������ٳ�����ͨ������ʵ���ȷ������B�ijɷ֣��뽫�±���д������
ʵ�鲽�� | ���� | �йط�Ӧ�Ļ�ѧ����ʽ |
ȡ��������B���μ�_____ | �����ݲ��� | _____ |
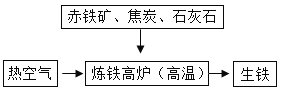
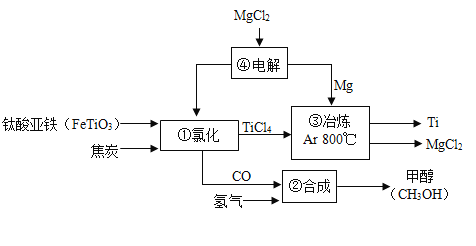
��3����ͼ�ǹ�ҵ����ʾ��ͼ�����У���̿��������ȼ���ṩ������_____���������ɵĻ�ѧ����ʽΪ_____��
��4��ij�������÷���м���������Ӧ����ȡ�������������з�����49t��H2SO4����������Ϊ10%��������������м��Ӧ������������������������_____


 B.
B. C.
C. D.
D.
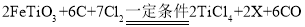 ����X�Ļ�ѧʽΪ_____��
����X�Ļ�ѧʽΪ_____��