��Ŀ����
����Ŀ��ij��ѧ����С���ͬѧ���������������顣���dz�ȡ�����ȫ��ͬ������Ϊ2.84g��4.26g��6.23g�����ݸ���(���������ֻ������̼)���ֱ����100gϡ�����н���ʵ��(ʵ��I����)����ַ�Ӧ���õ���ʵ�����ݻ��Ƴ�����ͼ��
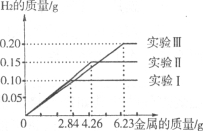
�Իش�(��������ȷ��0��1��)��
��1��д��ʵ�����йصĻ�ѧ����ʽ��
��2��ʵ��I�����μӷ�Ӧ������Ϊ ��
��3��ϡ������ȫ�μӷ�Ӧ��ʵ���У���Ӧ����Һ�����ʵ����������Ƕ��١�
���𰸡���1��Fe+H2SO4=FeSO4+H2�� ��2��2.8g ��3��14.4%
��������
�����������1������ϡ���ᷴӦ�ķ���ʽΪ:Fe+H2SO4=FeSO4+H2��
�����ݺ�ͼ������ж�ʵ����е�������ȫ��Ӧ������ʱʣ������������Ϊ0.2g�����ݻ�ѧ����ʽ��Fe+H2SO4=FeSO4+H2����H2��Fe��FeSO4��������ϵ���ɷֱ����Fe��FeSO4����������һ�������ʵ��I�����μӷ�Ӧ�������Լ���Ӧ����Һ�����ʵ���������
�⣺ ��ʵ����вμӷ�Ӧ��Fe������Ϊx�����ɵ�FeSO4������Ϊy
Fe + H2SO4 = FeSO4 + H2��
56 152 2
x y 0.2g
��2��56:2=x��0.2g x=5.6g
��3��152:2=y��0.2g y=15.2g
��Ӧ����Һ������=5.6g+100g-0.2g=105.4g
��Ӧ����Һ�����ʵ���������=15.2g/105.4g��100%=14.4%

 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�����Ŀ��ijͬѧΪ�˲ⶨNaCl��MgCl2����������MgCl2��������������������ʵ�飺��80g������������ˮ�����Һ��ƽ���ֳ��ķݣ��ֱ������ͬ����������NaOH��Һ���������ʵ�����ݣ�
ʵ����� | һ | �� | �� | �� |
���������������g�� | 20 | 20 | 20 | 20 |
����NaOH��Һ��������g�� | 20 | 40 | 60 | 80 |
���ɳ�����������g�� | 2.9 | m | 8.7 | 8.7 |
�ʣ�
��1������m��ֵΪ ����2�֣�
��2��ԭ����������MgCl2�����������Ƕ��٣���Ҫ��д��������̣�
����Ŀ�������װ�г�ʹ��һ�ִ�װ��������Ʒ��Ϊ��504˫�����������ǩ����ͼ��ʾ��ͬѧ�Ƕ�һ�����õġ�504˫������������Ʒ�ܺ��棬���ʵ�����̽����
Ʒ����504˫���� �ɷ֣����ۡ���ʯ�ҵ� |
��������⡿���ù���ijɷ���ʲô��
���������ϡ������Ȼ�����Һ�ڳ����·�����Ӧ�����Ȼ�������
���������롿���ù����п��ܺ���Fe��Fe2O3��CaO��Ca��OH��2��CaCO3�����ù����п��ܺ���Ca��OH��2��ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
��ʵ��̽��1��
��ͬѧ�ķ�����
ʵ����� | ʵ������ | ʵ����� |
��1��ȡ������������Թ��У�����������ˮ�ܽ⣬���ú�ȡ�ϲ���Һ�μ���ɫ��̪��Һ | �����ܽ�ʱ�Թ���ڷ��̣��Թܵײ��в������Һ��죮 | ������һ������ ���������ƣ� |
��2����ȡ������������Թ��У��μ������� �� | ��������ʧ���д�����ɫ����������õ�dz��ɫ��Һ�� | ������һ������ �� һ������Fe2O3 |
��3�������裨2���в���������ͨ�뵽�����ʯ��ˮ�� | ������һ������CaCO3 |
��ʵ�����ɡ�
��1����ͬѧ��Ϊ��ͬѧ��ʵ���в��ܵó�һ����Ca��OH��2�Ľ��ۣ������� ��
��2����ͬѧ��Ϊ��ͬѧ��ʵ�鲢���ܵó�һ������Fe2O3�Ľ��ۣ������� ��
��ʵ��̽��2��
�ҡ���ͬѧ�������ʵ�鷽��������֤��
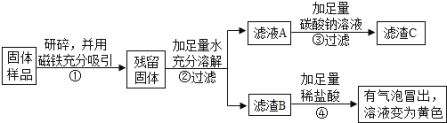
��1�����з�Ӧ�Ļ�ѧ����ʽ�� ��
��2���ҡ���ͬѧʵ�������ܵó�������Ʒ��һ�������� ������һ�����ʲ���ȷ������������ ��
��3���ҡ���ͬѧ�־���ʵ�����������������к������ʵ�������Ϊ1.6g������B��CaCO3������Ϊ1.0g������C������Ϊ1.0g��
��ʵ����ۡ��ۺ�����ʵ�鼰�������ݣ����ù���ijɷ��� ��