��Ŀ����
���������1��ijƷ���������Ƿ���̼��ƣ�
���������ϡ��������費��ϡ���ᷴӦ
��ʵ�鷽����
��ȡ�����������Թ��У��������ϡ���ᣬ�������ݣ�������ͨ�����ʯ��ˮ�У�ʯ��ˮ����ǣ�֤��Ħ�����к���
��ȡʵ����е���Һ������������Һ�����ɰ�ɫ����-����ƣ���д���÷�Ӧ�Ļ�ѧ����ʽ
���������2����������̼��Ƶĺ����Ƕ��٣�
��ʵ�鲽�衿
��1����ͼ���Ӻ�װ�ú���һ�����ԵĴ�����Ϊ
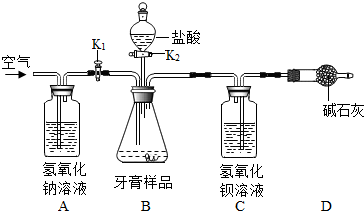
��2���������Ӻ�װ�ã���
��3����Bװ���м���������Ʒ8.00�ˣ�
��4��
��5��
��6����Cװ���еĹ���������ˡ�
��7���ظ�ʵ�飮
����֪��Ʒ�������е�Ħ����Ϊ̼��ƣ��������ɷֲ������ᷴӦ��װ�����Լ�����������
��ʵ�����ݡ��ظ�ʵ�飬3�����ݼ�¼���£�
| ʵ����� | ʵ��1 | ʵ��2 | ʵ��3 |
| Cװ���г���������g�� | 3.93 | 3.94 | 3.95 |
��1�����������ܷ���������ƽ��ã�
��2����û��Aװ�ã���ⶨ�����
��3��Dװ�õ�������
��4�����ϱ����ݣ������������Ʒ��̼��Ƶĺ����Ƕ��٣�
д��������̣�
��ʵ�鷴˼��
��1���ظ�ʵ�飬ȡƽ��ֵ����������
��2�����и����ʩ�У�����߲ⶨȷ�ȵ���
a����װ��A��B֮������ʢ��Ũ�����ϴ��ƿ
b����װ��B��C֮������ʢ�б���̼�����Ƶ�ϴ��ƿ
c�������μ�ϡ���ᣮ
��1��ϴ��װ���е���Ӧ�����̳���
��2��ʵ��ǰӦ���װ�õ������ԣ���ֹװ��©����
��4������װ�����ԭ����ʵ��Ŀ�ķ������
��5���ٴδ�K1���ر�K2������ͨ��һ��ʱ�������ʹ���ɵĶ�����̼��ַ�Ӧ��
��6����Cװ���еĹ���������ˡ�ϴ�ӡ���ɺ������������ȷ��
��ʵ����������ݴ�����
��1��������ƽ�ľ�ȷ�ȷ������
��2��Aװ�õ����������տ����еĶ�����̼����ֹ���������ȷ��
��3��Dװ�õ����������տ����еĶ�����̼����ֹ���������ȷ��
��4�������������ɳ������ݵ�ƽ��ֵ�����㷴Ӧ�Ķ�����̼������������������μӷ�Ӧ��̼��Ƶ�������
��ʵ�鷴˼��
��1����β���������ƽ��ֵ��ʵ��ij��÷������ɼ�����
��2����װ��B��C֮������ʢ�б���̼�����Ƶ�ϴ��ƿ�����Է�ֹ�ӷ������Ȼ�������Ƚ���Cװ�ö�ʹʵ������ȷ�������μ�ϡ�����ʹ���ɵĶ�����̼��Ӧ��ȫ��ʹ���������ȷ��
��ʵ����е���Һ�к��������ӣ�����������Һ�����ɰ�ɫ����-����ƣ�˵������Һ�к��и����ӣ���Ӧ�Ļ�ѧ����ʽ�ǣ�NH4��2C2O4+CaCl2=CaC2O4��+2NH4Cl��
��1��C�����������շ�Ӧ���ɵĶ�����̼��װ���е���Ӧ�����̳���
��2��ʵ��ǰӦ���װ�õ������ԣ���ֹװ��©����
��4���ر�K1����K2����������Ʒ�е���10%�����ᣬ�������������壬ֹͣ�μ����
��5���ٴδ�K1���ر�K2������ͨ��һ��ʱ�������ʹ���ɵĶ�����̼ȫ���ų�����ַ�Ӧ����C�в��ٲ���������
��6����Cװ���еĹ���������ˡ�ϴ�ӣ���ȥ������������ʣ�Ȼ���ɺ������������ȷ��
��ʵ����������ݴ�����
��1����ƽֻ�ܾ�ȷ��0.1g��������ƽ��������
��2��Aװ�õ����������տ����еĶ�����̼����û�и�װ�ã������C�����廹�п����еĶ�����̼��ʹ�������ƫ��
��3��Dװ�õ����������տ����еĶ�����̼����ֹ�����ж�����̼���룬ʹ���������ȷ��
��4�������������ɳ������ݵ�ƽ��ֵ�����㷴Ӧ�Ķ�����̼������������������������̼��Ƶ�����������������£�
��ʵ�鷴˼��
��1����β���������ƽ��ֵ��ʵ��ij��÷������ɼ�����
��2����װ��A��B֮������ʢ��Ũ�����ϴ��ƿ��������ˮ��������ˮ�������ڶ�����̼��Ӱ�죻��װ��B��C֮������ʢ�б���̼�����Ƶ�ϴ��ƿ�����Է�ֹ�ӷ������Ȼ�������Ƚ���Cװ�ö�ʹʵ������ȷ�������μ�ϡ�����ʹ���ɵĶ�����̼��Ӧ��ȫ��ʹ���������ȷ��
�ʴ�Ϊ����ʵ�鷽������̼������ڣ�NH4��2C2O4+CaCl2=CaC2O4��+2NH4Cl��
��1��Cװ���н���������Һ�����£���������¶����Ƥ�����ɣ�
��2�����װ�õ������ԣ�
��4���رգ������������ݲ�����
��5���ٴδ�K1���ر�K2������ͨ��һ��ʱ�������
��6��ϴ�ӣ�
��ʵ����������ݴ�����
��1�����ܣ���2��ƫ��3�����տ����еĶ�����̼����ֹ���ţ�
��4���������̼������Ϊx
Ba��OH��2+CO2�TBaCO3��+H2O
44 197
x 3.94g
| 44 |
| x |
| 197 |
| 3.94g |
x=0.88g
��̼��Ƶ�����Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
y 0.88g
| 100 |
| x |
| 44 |
| 0.88g |
y=2g
�𣺸�������Ʒ��̼��Ƶ�������2g��
��ʵ�鷴˼��
��1�����Լ�С����2��bc��

��Ӧ�����ǵ��ճ���������������еĹ�ϵ��
�������˵�θ����_________�����θҺ���ڹ��������θ�������ú�������������ҩ
��ɻ��ⲡʹ�������ƵĻ�ѧԭ��Ϊ���û�ѧ����ʽ��ʾ��_________________________��
�� ����ͷ��������ʱ�������������л��ᣬ����ͷǰ��ӽ������Ĵ����С�մ��
�������ʣ���������ò�����__________���壨�ѧʽ����ʹ��������ͷ���ɶ�ף�������ļ������ʹ��࣬�������Ż������еμ�����____________�����Ե�ζ�ϣ�������������ͷ���ɬ����ɫ���ơ�
�� δ�����ˮ��������ɬζ��������Ϊˮ���ﺬ�����ᡣ��ɬ�ķ���֮һ�ǣ�����
������ˮ����ʯ�һ���ʯ���飬�ñ仯�Ļ�ѧ����ʽ�� ___________________________��Ȼ���������ˮϡ�ͣ�ȡ�ϲ���ҹ������ˮ��5��6�켴�ɳ�ȥɬζ��
�� �˱����涣ҧ��Ƥ���������� ����������������Ƥ����ע���������������¡��������ұ����е������Ʒ________��______�����ţ�Ϳ�ڱ�ҧ��Ƥ���ϣ�ʹ�������ʧ��
| ���� | A | B | C | D |
| ����ˮ | ʳ�� | ���� | ʳ��ˮ | |
| pH | 10 | 3 | 9 | 7 |
�� �����پٳ�һ�������������г�����������������____________________��