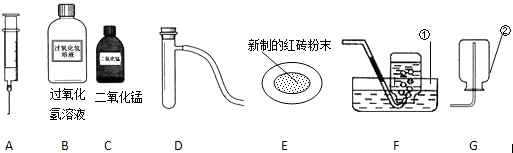
��Ŀ����
��ʵ�����п�ѡ����ͼ��ʾ������ҩƷ����ȡ������ͨ��ע������������ʱ���Թ��е������������Һ��
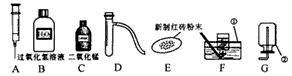
��ش������й����⣺
��1����д������٢���ŵĻ�ѧ�������ƣ� �� ���� ��
��2����ȡ���ռ�����Ӧѡ���װ��Ϊ �����ţ���
��3��������MnO2������������ѡ��ͼ����������������?
a. �������� ������ĸ��ţ���b. д����ȡ�����Ļ�ѧ����ʽ
��4�����ݳ���ѧϰ��֪ʶ�����ı�ҩƷ��������װ�û�����������ȡ�������Ǣ�
��д����ȡ������Ļ�ѧ����ʽ
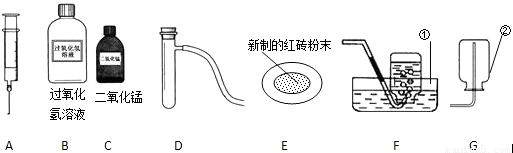
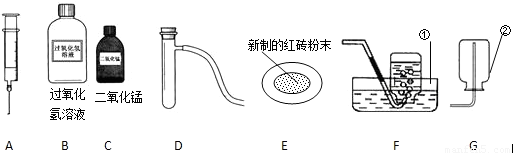
��1����д������٢���ŵĻ�ѧ�������ƣ� �� ���� ��
��2����ȡ���ռ�����Ӧѡ���װ��Ϊ �����ţ���
��3��������MnO2������������ѡ��ͼ����������������?
a. �������� ������ĸ��ţ���b. д����ȡ�����Ļ�ѧ����ʽ
��4�����ݳ���ѧϰ��֪ʶ�����ı�ҩƷ��������װ�û�����������ȡ�������Ǣ�
��д����ȡ������Ļ�ѧ����ʽ
��1���� ˮ�ۣ��� ����ƿ��
��2��ADF��
��3��E��2H2O2 2H2O+O2��
2H2O+O2��
��4����CO2��H2 ��CaCO3+2HCl===CaCl2+2H2O+CO2����Zn+H2SO4===ZnSO4+H2��
��2��ADF��
��3��E��2H2O2
 2H2O+O2��
2H2O+O2�� ��4����CO2��H2 ��CaCO3+2HCl===CaCl2+2H2O+CO2����Zn+H2SO4===ZnSO4+H2��

��ϰ��ϵ�д�
 Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д� ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д�
�����Ŀ