��Ŀ����
��09�ൺ����������Ҫ�Ľ������ϣ����������������й㷺����;�����챱�����˻����������������������˴����ĸ�����
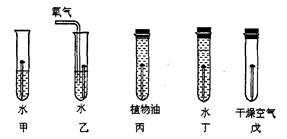
��1����������______________����������������
��2��ÿ�������ϸ����IJ����ܸߣ���������ʴҲ����������˾����ʧ�����ڿ�������ʴ��ʵ���������������е�__________��___________��ͬ���õĽ����
��3��Ϊ�˷�ֹ������ʴ�����dz������������Ϳˢ�����ͻ�������������ȸ��DZ���Ĥ�ķ�������Щ�������ܷ�ֹ��ʴ�Ĺ�ͬԭ����_________________________��
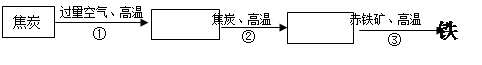
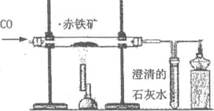
��4�����������Խ�̿����������Ҫ�ɷ�������������������Ϊ��Ҫԭ����������Ӧ�������£�
��̿ CO2
CO2  CO
CO  Fe
Fe
��д���ڢ۲���Ӧ�Ļ�ѧ����ʽ��_________________________________________��
��5����������Ĵ������ޣ����Ҳ���������Ŀǰ����������50%���ϵķϸ����õ��������ã���Ŀ����_____________________________����������ɿ����Լ������Դ������
��1����������______________����������������
��2��ÿ�������ϸ����IJ����ܸߣ���������ʴҲ����������˾����ʧ�����ڿ�������ʴ��ʵ���������������е�__________��___________��ͬ���õĽ����
��3��Ϊ�˷�ֹ������ʴ�����dz������������Ϳˢ�����ͻ�������������ȸ��DZ���Ĥ�ķ�������Щ�������ܷ�ֹ��ʴ�Ĺ�ͬԭ����_________________________��
��4�����������Խ�̿����������Ҫ�ɷ�������������������Ϊ��Ҫԭ����������Ӧ�������£�
��̿
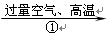 CO2
CO2  CO
CO 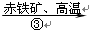 Fe
Fe ��д���ڢ۲���Ӧ�Ļ�ѧ����ʽ��_________________________________________��
��5����������Ĵ������ޣ����Ҳ���������Ŀǰ����������50%���ϵķϸ����õ��������ã���Ŀ����_____________________________����������ɿ����Լ������Դ������
��1������� ��2��ˮ���� ���� ��3����������
��4��3CO + Fe2O3 2Fe + 3CO2 ��5����Լ������Դ
2Fe + 3CO2 ��5����Լ������Դ
��4��3CO + Fe2O3
 2Fe + 3CO2 ��5����Լ������Դ
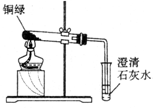
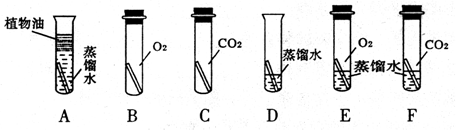
2Fe + 3CO2 ��5����Լ������Դ��������������������Ǹ�����������ˮͬʱ�Ӵ�����ֹ��������ķ����У��ڽ�������Ϳһ������ڽ��������һ�㲻���õĽ����ȣ����������غ㶨�ɿ�����д��ѧ����ʽ��
��𣺽⣺��1�����������к���̼������һЩ���ʵĻ����������
��2�����������Ǹ�����ˮ������ͬʱ���õĽ�������������ˮ������
��3������������ˮ���Է�ֹ�������⣮�������������ˮ��
��4����������һ����̼��Ӧ���������Ͷ�����̼�����3CO+Fe2O3 2Fe+3CO2
2Fe+3CO2
��5���ϸ����õ��������ã���Ŀ���ǽ�Լ������Դ�������Լ������Դ��
��𣺽⣺��1�����������к���̼������һЩ���ʵĻ����������
��2�����������Ǹ�����ˮ������ͬʱ���õĽ�������������ˮ������
��3������������ˮ���Է�ֹ�������⣮�������������ˮ��
��4����������һ����̼��Ӧ���������Ͷ�����̼�����3CO+Fe2O3
 2Fe+3CO2
2Fe+3CO2��5���ϸ����õ��������ã���Ŀ���ǽ�Լ������Դ�������Լ������Դ��

��ϰ��ϵ�д�
�����Ŀ