��Ŀ����
С��ͬѧ���ֳ���������ƿ�ޱ�ǩ�İ�ɫ��ĩ��������������մ��С�մ�Ϊ�˼�������ƿ��ɫ��ĩ��С�ȸ�ȡ��һС���������DZ��ΪA��B��Ȼ�����ʵ��̽����
���������ϡ��մ�ѧ��Ϊ̼���ƣ� 20��ʱ�ܽ��Ϊ21.6g����ˮ��Һ�ʼ��ԡ���
С�մ�ѧ��Ϊ̼�����ƣ� 20��ʱ�ܽ��Ϊ9.8g����ˮ��Һ�ļ��Ա�̼������Һ��������
��ʵ��̽��������������Ϣ��ѧ����֪ʶ��С�Ƚ��������µ�ʵ�飺

A B
��1��20��ʱ����A��B�������ʸ�6g�ֱ���뵽50g����ˮ��,�а�ɫ����ʣ����� ��
��2����pH��ֽ���A����ҺpH=10��B����ҺpH=8����A��ĩ�� ��
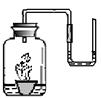
 ��3��С��ͨ�����㷢�֣���������̼������̼��������������ϡ���ᷴӦ�� �����Ķ�����̼���࣬��������������ʵ�鷽������U���зֱ���������A��B���ֹ��壬����֧ע�����зֱ���������ϡ���ᣬ��ע��U���У���д��̼��������ϡ���ᷴӦ�Ļ�ѧ����ʽ�� �� ʵ����Ҫ�۲�������� ��
��3��С��ͨ�����㷢�֣���������̼������̼��������������ϡ���ᷴӦ�� �����Ķ�����̼���࣬��������������ʵ�鷽������U���зֱ���������A��B���ֹ��壬����֧ע�����зֱ���������ϡ���ᣬ��ע��U���У���д��̼��������ϡ���ᷴӦ�Ļ�ѧ����ʽ�� �� ʵ����Ҫ�۲�������� ��
����˼��ߡ�(4)С������������ʵ�鷽����һ��̽��̼���ƺ�̼�����������ᷴӦ�Ŀ���,�����Ʒ����ĽǶ�˵��һ���Ƿ����
��
���������ϡ��մ�ѧ��Ϊ̼���ƣ� 20��ʱ�ܽ��Ϊ21.6g����ˮ��Һ�ʼ��ԡ���
С�մ�ѧ��Ϊ̼�����ƣ� 20��ʱ�ܽ��Ϊ9.8g����ˮ��Һ�ļ��Ա�̼������Һ��������
��ʵ��̽��������������Ϣ��ѧ����֪ʶ��С�Ƚ��������µ�ʵ�飺

A B
��1��20��ʱ����A��B�������ʸ�6g�ֱ���뵽50g����ˮ��,�а�ɫ����ʣ����� ��
��2����pH��ֽ���A����ҺpH=10��B����ҺpH=8����A��ĩ�� ��
 ��3��С��ͨ�����㷢�֣���������̼������̼��������������ϡ���ᷴӦ�� �����Ķ�����̼���࣬��������������ʵ�鷽������U���зֱ���������A��B���ֹ��壬����֧ע�����зֱ���������ϡ���ᣬ��ע��U���У���д��̼��������ϡ���ᷴӦ�Ļ�ѧ����ʽ�� �� ʵ����Ҫ�۲�������� ��
��3��С��ͨ�����㷢�֣���������̼������̼��������������ϡ���ᷴӦ�� �����Ķ�����̼���࣬��������������ʵ�鷽������U���зֱ���������A��B���ֹ��壬����֧ע�����зֱ���������ϡ���ᣬ��ע��U���У���д��̼��������ϡ���ᷴӦ�Ļ�ѧ����ʽ�� �� ʵ����Ҫ�۲�������� ������˼��ߡ�(4)С������������ʵ�鷽����һ��̽��̼���ƺ�̼�����������ᷴӦ�Ŀ���,�����Ʒ����ĽǶ�˵��һ���Ƿ����
��
��1��̼������ ��2��̼���� ��3��̼������ NaHCO3+HCl=NaCl+CO2��+H2O
�����������͵Ĵ�С ��4�������������ù���������ȣ���������û��˵�������ͬ������������ͬ��2�֣�
�����������͵Ĵ�С ��4�������������ù���������ȣ���������û��˵�������ͬ������������ͬ��2�֣�
��1������Ŀ���Ͽ�֪��0��ʱ̼�������ܽ����9.8�ˣ�̼���Ƶ��ܽ����21.6�ˣ�20��ʱ����50��ˮ�м���6�˸ð�ɫ��ĩ����̼�����Ƶ��ܽ�ȿ�֪50��ˮ������ܽ�4.9��̼�����ƣ�����ɫ����ʣ�࣬û����ȫ�ܽ��Ӧ����̼�����ƣ�
��2���������⣺̼�����Ƶ�ˮ��Һ�ļ��Ա�̼������Һ������A����ҺpH=10��B����ҺpH=8��A�ļ���ǿ������A��̼���ƣ�
��3��������������̼���ơ�̼��������ȫ�μӷ�Ӧ��
Na2CO3+2HCl�T2NaCl+CO2��+H20��
106 44
NaHCO3+2HCl�T2NaCl+CO2��+H20��
84 44
�ɷ�Ӧ�Ļ�ѧ����ʽ���ʼ��������ϵ��֪��106��̼���ƿɷ�Ӧ����44�˶�����̼����84��̼�����ƿɷ�Ӧ����44�˶�����̼�����Ե�������̼������̼��������������ϡ���ᷴӦ��̼���������ɵĶ�����̼�������ࣻʵ����Ҫ�۲�������ǣ������������͵Ĵ�С��
��4��Ҫ��һ��̽��̼���ƺ�̼�����������ᷴӦ�Ŀ������������ù���������ȣ��������������ͬ����������ҲҪ��ͬ��������������ʵ�鷽����һ��̽��̼���ƺ�̼�����������ᷴӦ�Ŀ����Dz������ġ�
��2���������⣺̼�����Ƶ�ˮ��Һ�ļ��Ա�̼������Һ������A����ҺpH=10��B����ҺpH=8��A�ļ���ǿ������A��̼���ƣ�
��3��������������̼���ơ�̼��������ȫ�μӷ�Ӧ��
Na2CO3+2HCl�T2NaCl+CO2��+H20��
106 44
NaHCO3+2HCl�T2NaCl+CO2��+H20��
84 44
�ɷ�Ӧ�Ļ�ѧ����ʽ���ʼ��������ϵ��֪��106��̼���ƿɷ�Ӧ����44�˶�����̼����84��̼�����ƿɷ�Ӧ����44�˶�����̼�����Ե�������̼������̼��������������ϡ���ᷴӦ��̼���������ɵĶ�����̼�������ࣻʵ����Ҫ�۲�������ǣ������������͵Ĵ�С��
��4��Ҫ��һ��̽��̼���ƺ�̼�����������ᷴӦ�Ŀ������������ù���������ȣ��������������ͬ����������ҲҪ��ͬ��������������ʵ�鷽����һ��̽��̼���ƺ�̼�����������ᷴӦ�Ŀ����Dz������ġ�

��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д�
�����Ŀ