��Ŀ����
 ʳƷ�뽡������Դ�뻷�������ǹ�ͬ��ע��������⣮
ʳƷ�뽡������Դ�뻷�������ǹ�ͬ��ע��������⣮
��1������Ӫ���ḻ����������ı������ܣ������и������ۡ�ά����C��ά����B�������ơ����ȣ�
������ġ����������ơ���ָ________������ĸ��ţ���
A��ԭ�ӡ��� B�����ӡ� C��Ԫ�ء� D������
������ȱ���������ᵼ��________��֢��
���������ܸ������ṩ������������________��
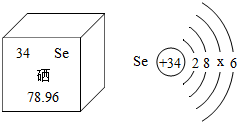
����Ԫ�ر���Ϊ����������������Ԫ�صIJ�����Ϣ��ͼ������˵����ȷ����________������ĸ��ţ���
A�������ڽ���Ԫ��
B��һ����ԭ������34������
C������ԭ�ӽṹʾ��ͼ��x=18
D����Ԫ�ص����ԭ������Ϊ78.96g
��2����Ȼ��������ʹ�õ�ȼ��֮һ������Ҫ�ɷ�ȼ�շ�Ӧ�Ļ�ѧ����ʽ��________��
��3��������̼���������ЧӦ����Ҫ���壬�ӡ���̼���ĽǶȷ�����Ӧ�������ٶ�����̼���ŷţ�
����������ú��ʯ���У��������ȼ����________��
�ڽ��ŷų��Ķ�����̼���ղ�ת��Ϊ�������õ������ǿ�ѧ���о��ķ��������պ���������Ƿ��ж�����̼�����ݷ�Ӧ�Ļ�ѧ����ʽ��________��
�⣺��1��������ġ����������ơ���ָ�����е�Ԫ�أ����������ӡ������ԭ�ӵ���ʽ���ڣ�
������ȱ���������ᵼ��ƶѪ����
�������еĵ����ܸ������ṩ������
�ܿɸ��ݺ����ص㣬��Ԫ�����ǽ���Ԫ�أ���A��������ԭ��������34������ԭ������=����������֪����������34������B��ȷ������ԭ����������=�������������֪������������34����������֪x=34-2-8-6=18������C��ȷ�����ԭ�������ĵ�λ��1����D����
��2����Ȼ������Ҫ�ɷ��Ǽ��飬����������ڵ�ȼ�����������ɶ�����̼��ˮ������ʽ�ǣ�CH4+2O2 CO2+2H2O��
CO2+2H2O��
��3�����������з��ȶࡢ��Դ�㡢��������Ⱦ���ص㣬���������ȼ�ϣ�
�ڼ��������̼�ó����ʯ��ˮ��������̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ������ʽ�ǣ�Ca��OH��2+CO2=CaCO3��+H2O��
�ʴ�Ϊ��
��1����C�� ��ƶѪ�� �۵��ۣ� ��BC��
��2��CH4+2O2 CO2+2H2O��
CO2+2H2O��
��3���������� ��Ca��OH��2+CO2=CaCO3��+H2O
��������1��������ġ����������ơ���ָ�����е�Ԫ�أ����������ӡ������ԭ�ӵ���ʽ���ڣ�
������ȱ���������ᵼ��ƶѪ����
�������еĵ����ܸ������ṩ������
�ܸ���Ԫ�ص����ࡢ�����������ԭ�������ĵ�λ�������
��2��������Ȼ��ȼ�յķ�Ӧԭ����д����ʽ��
��3�����������з��ȶࡢ��Դ�㡢��������Ⱦ���ص㣬���������ȼ�ϣ�
�����ݼ��������̼�ó����ʯ��ˮ��������̼���������Ʒ�Ӧԭ����д����ʽ��
���������⿼��֪ʶ˼ά��Ƚϴ�������ѧ֪ʶ����������ϸ�ķ���������ȷ���
������ȱ���������ᵼ��ƶѪ����
�������еĵ����ܸ������ṩ������
�ܿɸ��ݺ����ص㣬��Ԫ�����ǽ���Ԫ�أ���A��������ԭ��������34������ԭ������=����������֪����������34������B��ȷ������ԭ����������=�������������֪������������34����������֪x=34-2-8-6=18������C��ȷ�����ԭ�������ĵ�λ��1����D����
��2����Ȼ������Ҫ�ɷ��Ǽ��飬����������ڵ�ȼ�����������ɶ�����̼��ˮ������ʽ�ǣ�CH4+2O2
 CO2+2H2O��
CO2+2H2O����3�����������з��ȶࡢ��Դ�㡢��������Ⱦ���ص㣬���������ȼ�ϣ�
�ڼ��������̼�ó����ʯ��ˮ��������̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ������ʽ�ǣ�Ca��OH��2+CO2=CaCO3��+H2O��
�ʴ�Ϊ��
��1����C�� ��ƶѪ�� �۵��ۣ� ��BC��
��2��CH4+2O2
 CO2+2H2O��
CO2+2H2O����3���������� ��Ca��OH��2+CO2=CaCO3��+H2O
��������1��������ġ����������ơ���ָ�����е�Ԫ�أ����������ӡ������ԭ�ӵ���ʽ���ڣ�
������ȱ���������ᵼ��ƶѪ����
�������еĵ����ܸ������ṩ������
�ܸ���Ԫ�ص����ࡢ�����������ԭ�������ĵ�λ�������
��2��������Ȼ��ȼ�յķ�Ӧԭ����д����ʽ��
��3�����������з��ȶࡢ��Դ�㡢��������Ⱦ���ص㣬���������ȼ�ϣ�
�����ݼ��������̼�ó����ʯ��ˮ��������̼���������Ʒ�Ӧԭ����д����ʽ��
���������⿼��֪ʶ˼ά��Ƚϴ�������ѧ֪ʶ����������ϸ�ķ���������ȷ���

��ϰ��ϵ�д�
�����Ŀ
 ��2013?��������ģ��ʳƷ�뽡������Դ�뻷�������ǹ�ͬ��ע��������⣮
��2013?��������ģ��ʳƷ�뽡������Դ�뻷�������ǹ�ͬ��ע��������⣮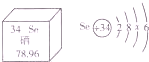 ʳƷ�뽡������Դ�뻷�������ǹ�ͬ��ע��������⣮
ʳƷ�뽡������Դ�뻷�������ǹ�ͬ��ע��������⣮ ʳƷ�뽡������Դ�뻷�������ǹ�ͬ��ע��������⣮
ʳƷ�뽡������Դ�뻷�������ǹ�ͬ��ע��������⣮
