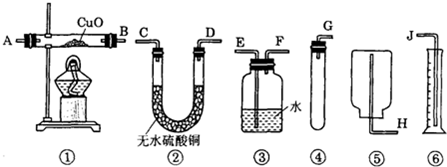
��Ŀ����
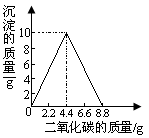
��5�֣�ijʵ��С����һ������ʯ��ˮ��ͨ��CO2������������������ͨ��CO2 �����Ĺ�ϵ����ͼ��ʾ����֪��Ӧ��CaCO3+H2O+CO2=Ca(HCO3)2��Ca(HCO3)2������ˮ��
��1��������ʵ������п��ܹ۲쵽������������2�֣���Ϊʹ��Һ������������ʵ�������Ϊ���ֵ������������Ӧͨ��CO2 ������������
��������CO2������ˮ�е��ܽ⣩
��2�������ͨCO2����ǰʯ��ˮ�����ʵ�����������д��������̣�2�֣�

��1��������ʵ������п��ܹ۲쵽������������2�֣���Ϊʹ��Һ������������ʵ�������Ϊ���ֵ������������Ӧͨ��CO2 ������������
��������CO2������ˮ�е��ܽ⣩
��2�������ͨCO2����ǰʯ��ˮ�����ʵ�����������д��������̣�2�֣�
���Ȼ��ǣ������ 8.8g (2) 7.4g
���������(1) һ������ʯ��ˮ��ͨ��CO2��������Ӧ��CO2 +Ca(OH)2 ==CaCO3��+ H20������ͨ��CO2���ַ�����Ӧ����CaCO3+H2O+CO2=Ca(HCO3)2����ʵ������п��ܹ۲쵽�������Ȼ��ǣ�����壬ͨ���۲�ͼ�е����ݣ�Ϊʹ��Һ������������ʵ�������Ϊ���ֵ������������Ӧͨ��CO2 ������8.8g
(2)����ͼ�е����ݣ���֪��ͨ���CO2 ������4.4gʱ��ǡ����ʯ��ˮ������Ca(OH)2��ȫ��Ӧ���ٸ��ݷ�Ӧ��CO2 +Ca(OH)2 ==CaCO3��+ H20��CO2��Ca(OH)2��������ϵ���������ʯ��ˮ�����ʵ�����
�ƽ⣺�����ʯ��ˮ������Ca(OH)2������Ϊx
CO2+ Ca(OH) 2 ="===" CaCO3��+ H2O
44 74
4.4g x
44/74=4.4g/x x=7.4g
�𣺳���ʯ��ˮ������Ca(OH)2������Ϊ7.4g

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ