��Ŀ����
����Ŀ����1����ѧ������ѧϰ���о���ѧ����Ҫ���ߣ���д����������Ҫ��Ļ�ѧ���
������Һ�ж����е�������_______�� ���������������____________��
�������³�Һ̬���ӷ��ļ�_____�� �����������Ƽ�����_________��
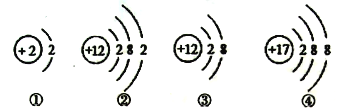
��2����ͼ��ijƷ�Ƶ緹�ҵ�ʾ��ͼ��

�����������У����ںϽ����_________����һ�ּ��ɣ���
���緹�ҵ�������������ģ�����ʱ����________���ϣ��� ���������������ȹ���������
�����кܺõĿ���ʴ�ԣ���ԭ���ǣ��û�ѧ����ʽ�ش�____________________________��
����������ĸ��Ӵ����ڸ����������ĵ�ˮ��_________������Ӳˮ��������ˮ������
���𰸡� H+ SO2��NO2 NH3��H2O CaCO3 ���Ͻ����� �ȹ��� 4Al+3O2�T2Al2O3 ��ˮ
����������1�������ǵ���ʱ�����������ȫ���������ӵĻ������������Һ�ж����е�������Ϊ��H+��
�ڶ��������������������������Ҫ���ʣ��仯ѧʽΪ��SO2��NO2��
�۰�ˮ��һ���ӷ��ij����³�Һ̬�ļ�仯ѧʽΪ��NH3��H2O��
��̼��ƺ��и�Ԫ�أ�����θ�ᣨ��Ҫ�ɷ������ᣩ��Ӧ���ɿ����Եĸ��Σ����������գ����������Ƽ�����ѧʽΪ��CaCO3��
��2���ٺϽ���ָ��һ�ֽ����м����ۺ�����������ǽ������γɵľ��н������Ե����ʣ��������Ͻ�Ͳ�������ںϽ�
�ڵ緹�ҵ���������ȹ��������Ƴɵģ���ֹ�����ۻ����Σ�
������������Ӧ��������������Ӧ�Ļ�ѧ����ʽΪ��4Al+3O2�T2Al2O3��
�ܰ�������ĸ��Ӵ����ڸ��ϵ�������ˮ�Ǿ�����������ˮ��

 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д�