��Ŀ����
��1������H2��2H ��
��H+���ַ��ţ��京�������б�����һ�µ��ǣ��������գ�
A����ʾ2����ԭ�ӵ��� �� B����ʾ+1����Ԫ�ص��� ��
C����ʾ��1����λ����ɵ������ӵ��� ��
D����ʾ1��������к���2����ԭ�ӵ��� ��
��2����A������B����ʯ��C������D����������E������F���ɱ�G���ƾ�H��̼����藺��������У�ѡ������������������ʣ�����Ҫ�ɷ֣����û�ѧʽ��գ�
�ٿ������˹�������� �� ��ҽ������������Σ�ز��˵��� ��
��δ�������������ȼ���� �� �ܳ�������ʳƷ��������� ��
����Ⱦ�������γ�������� �� ����Ȼ������Ҫ�ɷ��� ��
�߿����������ɼ���Ҳ�������ʵ��� �� ��ʵ������õ�ȼ���� ��
| +1 |
| H |
A����ʾ2����ԭ�ӵ���
C����ʾ��1����λ����ɵ������ӵ���
D����ʾ1��������к���2����ԭ�ӵ���
��2����A������B����ʯ��C������D����������E������F���ɱ�G���ƾ�H��̼����藺��������У�ѡ������������������ʣ�����Ҫ�ɷ֣����û�ѧʽ��գ�
�ٿ������˹��������
��δ�������������ȼ����
����Ⱦ�������γ��������
�߿����������ɼ���Ҳ�������ʵ���
���㣺��ѧ���ż�����Χ���ֵ�����,��ѧʽ����д������
ר�⣺��ѧ����������غ㶨��
��������1��A��ԭ�ӵı�ʾ��������Ԫ�ط�������ʾһ��ԭ�ӣ���ʾ�����ԭ�ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�
B�����ϼ۵ı�ʾ���������仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�
C�����ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�
D���ɷ��ӹ��ɵ����ʣ��仯ѧʽ�ܱ�ʾһ�����ӣ�
��2�����ȸ�������ȷ�����ʵĻ�ѧ���ƣ�Ȼ�������д��ѧʽ�ķ����Ͳ���д�����ʵĻ�ѧʽ���ɣ�
B�����ϼ۵ı�ʾ���������仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�
C�����ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�
D���ɷ��ӹ��ɵ����ʣ��仯ѧʽ�ܱ�ʾһ�����ӣ�
��2�����ȸ�������ȷ�����ʵĻ�ѧ���ƣ�Ȼ�������д��ѧʽ�ķ����Ͳ���д�����ʵĻ�ѧʽ���ɣ�
����⣺��1��A����ԭ�ӵı�ʾ��������Ԫ�ط�������ʾһ��ԭ�ӣ���ʾ�����ԭ�ӣ�������Ԫ�ط���ǰ������Ӧ�����֣���2����ԭ�ӱ�ʾΪ��2H��
B���ɻ��ϼ۵ı�ʾ���������仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�+1����Ԫ�ؿɱ�ʾΪ��
��
C�������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ���H+�ɱ�ʾ��1����λ����ɵ������ӣ�
D���ɷ��ӹ��ɵ����ʣ��仯ѧʽ�ܱ�ʾһ�����ӣ�H2�е�2��ʾ1��������к���2����ԭ�ӣ�
��2���ٸɱ�������Ķ�����̼������ʱ���մ������ȣ��������˹����꣬�仯ѧʽΪ��CO2��
�������ܹ���������ҽ������������Σ�ز��ˣ��仯ѧʽΪ��O2��
������ȼ�ղ�����ˮ������Ⱦ����δ�������������ȼ�ϣ��仯ѧʽΪ��H2��
����ʯ������ˮ��Ӧ�����������ƣ���������ʳƷ��������仯ѧʽΪ��CaO��
�ݶ���������Ⱦ�������γ����꣬�仯ѧʽΪ��SO2��
����Ȼ������Ҫ�ɷ��Ǽ��飬�仯ѧʽΪ��CH4��
��̼����刺����������ɼ���Ҳ�������ʣ��仯ѧʽΪ��NH4HCO3��
��ʵ������õ�ȼ���Ǿƾ����仯ѧʽΪ��C2H5OH��
�ʴ�Ϊ����1��A���ڣ�B���ۣ�C���ܣ�D���٣���2����CO2����O2����H2����CaO����SO2����CH4����NH4HCO3����C2H5OH��
B���ɻ��ϼ۵ı�ʾ���������仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�+1����Ԫ�ؿɱ�ʾΪ��
| +1 |
| H |
C�������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ���H+�ɱ�ʾ��1����λ����ɵ������ӣ�
D���ɷ��ӹ��ɵ����ʣ��仯ѧʽ�ܱ�ʾһ�����ӣ�H2�е�2��ʾ1��������к���2����ԭ�ӣ�
��2���ٸɱ�������Ķ�����̼������ʱ���մ������ȣ��������˹����꣬�仯ѧʽΪ��CO2��
�������ܹ���������ҽ������������Σ�ز��ˣ��仯ѧʽΪ��O2��
������ȼ�ղ�����ˮ������Ⱦ����δ�������������ȼ�ϣ��仯ѧʽΪ��H2��
����ʯ������ˮ��Ӧ�����������ƣ���������ʳƷ��������仯ѧʽΪ��CaO��
�ݶ���������Ⱦ�������γ����꣬�仯ѧʽΪ��SO2��
����Ȼ������Ҫ�ɷ��Ǽ��飬�仯ѧʽΪ��CH4��
��̼����刺����������ɼ���Ҳ�������ʣ��仯ѧʽΪ��NH4HCO3��
��ʵ������õ�ȼ���Ǿƾ����仯ѧʽΪ��C2H5OH��
�ʴ�Ϊ����1��A���ڣ�B���ۣ�C���ܣ�D���٣���2����CO2����O2����H2����CaO����SO2����CH4����NH4HCO3����C2H5OH��
�����������ѶȲ�����Ҫ����ͬѧ�ǶԳ�����ѧ���ԭ�ӷ��š����ӷ��š���ѧʽ�����ϼۡ����ӷ��ŵȣ�����д������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��ijС���Һ��Ϊֻ����һ�����ʵ���Һ�����������������̺������ʹ�����ǵ�ľ����ȼ�����壮������˵������ȷ���ǣ�������
| A����Ӧǰ��������̵Ļ�ѧ���ʲ��� |
| B�����������峣��ʱ����þ���������ʷ���������Ӧ |
| C�����������������ˮ���������ſ������ռ� |
| D���÷�Ӧ�Ļ�ѧ����ʽΪ2H2O2=H2O+O2 |
 A��B���ֹ������ʵ��ܽ����������ͼ��ʾ����ͼ�ش��������⣺
A��B���ֹ������ʵ��ܽ����������ͼ��ʾ����ͼ�ش��������⣺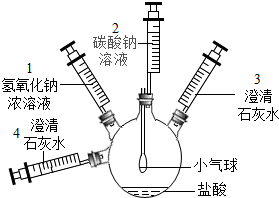
 ʵ��������һƿ��ǩ�������ɫҺ�壬��ͼ��ʾ����ƿ��ɫҺ����ʲô�أ�ʵ������ʦ���ߴ�ң���Һ��Ŀǰֻ���ǹ���������Һ������ˮ�е�һ�֣�
ʵ��������һƿ��ǩ�������ɫҺ�壬��ͼ��ʾ����ƿ��ɫҺ����ʲô�أ�ʵ������ʦ���ߴ�ң���Һ��Ŀǰֻ���ǹ���������Һ������ˮ�е�һ�֣�