��Ŀ����
��2000��12��18�ա������ձ�����ѧУ��ѧʵ����Ҳ����ȾԴ��һ��ָ����ȫ��������Ƶĸ�У����ѧ�Ļ�ѧʵ����ÿ�춼�ŷ��ųɷָ��ӵ���Ⱦ�Ϊ��ijѧУ��ѧ�о�С�����������ʵ�飬�Դ��Բ��Ը�����ʵ���ʵ���ң��ף�������ȫ����������ĺ��������裺��1��ȡ������ʵ������һ��û����Ⱦ��ʵ���ң��ң����У��������ȡ��������ѧʵ���ң��ף�������Ʒ�ķ�����______��
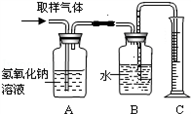
��2������ͼװ�ý�ȡ�õ��������ʵ�飬�ʣ�ͼ��װ��A��������______��װ��C��������______�������������к��ж���������д������Aװ���еķ�Ӧ�Ļ�ѧ����ʽ��______��
��3�����㣺��ȡ������Ϊ100mL��װ��C����Ϊ99.0mL����ʵ���ң��ף����������������庬��ԼΪ______%��
����ʽΪ�����������庬��=���������������/ȡ�������������×100%��
������a��һ������к�0.03%�Ķ�����̼������Ʒ����ʵ���ҷ�����������������ԼΪ______%
b����������ڽ��л�ѧʵ��ʱ������ʵ���Ҷ��������滷����Ⱦ��һ��������______��

���𰸡���������1�������ǻ�ѧʵ���ң��ף�������ֻҪ�������������弴�ɣ�
��2���������������������巴Ӧ���Ӷ��ﵽ�������������Ŀ�ģ�����Ͳ��ӵIJ���ʣ������壻
��3��a�������⣬��������ȥ��֪�������δ֪����b�ӱ����������н��
����𣺣�1������ؼ�����һ�������Ŀ�������ȡ��ѧʵ���ң��ף��Ŀ����������ܶ࣬�磺A��ʹ��ע���� B��ʹ��װ��ˮ��ƿ�ӣ�����ʵ���Һ�ȥˮ���øɲ����� C��ʹ������ �ʴ�Ϊ����һ������ƿװ��ˮ�õ�ʵ���ң��ף��а�ˮ������Ȼ����ϲ�Ƭ
��2������������һ�ּ�������������巴Ӧ���Ӷ��ﵽ�������������Ŀ�ģ�����Ͳ������B�ų���ˮ�������������������������������������������������������Ӧ�����������ƺ�ˮ���ʴ�Ϊ��������Ʒ�е��������壻�������������������������Ĵ�������� SO2+2NaOH=Na2SO3+H2O
��3��100mL����ͨ��װ��A��װ��C����Ϊ99.0mL��˵�����ٵ�1.0mL������ʵ���ң��ף������е����������壬������������ʽ�����֪ʵ���ң��ף������е����������庬��ԼΪ1%���ʴ�Ϊ��1%
�ٿ����еĶ�����̼���������壬���ԣ���ʵ���ҷ�����������������ԼΪ1%-0.03%=0.97%���ʴ�Ϊ��0.97%
�ڽ��л�ѧʵ��ʱ������ʵ���Ҷ��������滷����Ⱦ�����ӻ����ǶȽ�𣮹ʴ�Ϊ�����ҵ���Һ�����ӷ���
�������������Ի�ѧʵ����Ϊ���ж���Ƶļ�˼ά���š����ݿ��š����ۿ���Ϊһ����ۺ��Կ������⣬��һ���ѵõĺ��⣬���ܿ���ѧ���������������ܽ�������Ǩ����������������ѧ���Ĵ���˼ά��
��2���������������������巴Ӧ���Ӷ��ﵽ�������������Ŀ�ģ�����Ͳ��ӵIJ���ʣ������壻
��3��a�������⣬��������ȥ��֪�������δ֪����b�ӱ����������н��
����𣺣�1������ؼ�����һ�������Ŀ�������ȡ��ѧʵ���ң��ף��Ŀ����������ܶ࣬�磺A��ʹ��ע���� B��ʹ��װ��ˮ��ƿ�ӣ�����ʵ���Һ�ȥˮ���øɲ����� C��ʹ������ �ʴ�Ϊ����һ������ƿװ��ˮ�õ�ʵ���ң��ף��а�ˮ������Ȼ����ϲ�Ƭ
��2������������һ�ּ�������������巴Ӧ���Ӷ��ﵽ�������������Ŀ�ģ�����Ͳ������B�ų���ˮ�������������������������������������������������������Ӧ�����������ƺ�ˮ���ʴ�Ϊ��������Ʒ�е��������壻�������������������������Ĵ�������� SO2+2NaOH=Na2SO3+H2O
��3��100mL����ͨ��װ��A��װ��C����Ϊ99.0mL��˵�����ٵ�1.0mL������ʵ���ң��ף������е����������壬������������ʽ�����֪ʵ���ң��ף������е����������庬��ԼΪ1%���ʴ�Ϊ��1%
�ٿ����еĶ�����̼���������壬���ԣ���ʵ���ҷ�����������������ԼΪ1%-0.03%=0.97%���ʴ�Ϊ��0.97%
�ڽ��л�ѧʵ��ʱ������ʵ���Ҷ��������滷����Ⱦ�����ӻ����ǶȽ�𣮹ʴ�Ϊ�����ҵ���Һ�����ӷ���
�������������Ի�ѧʵ����Ϊ���ж���Ƶļ�˼ά���š����ݿ��š����ۿ���Ϊһ����ۺ��Կ������⣬��һ���ѵõĺ��⣬���ܿ���ѧ���������������ܽ�������Ǩ����������������ѧ���Ĵ���˼ά��

��ϰ��ϵ�д�
 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ
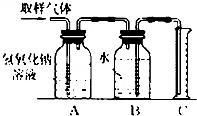 13����2000��12��18�ա������ձ�����ѧУ��ѧʵ����Ҳ����ȾԴ��һ��ָ����ȫ��������Ƶĸ�У����ѧ�Ļ�ѧʵ����ÿ�춼�ŷ��ųɷָ��ӵ���Ⱦ�Ϊ��ijѧУ��ѧ�о�С�����������ʵ�飬�Դ��Բ��Ը�����ʵ���ʵ���ң��ף�������ȫ����������ĺ�����
13����2000��12��18�ա������ձ�����ѧУ��ѧʵ����Ҳ����ȾԴ��һ��ָ����ȫ��������Ƶĸ�У����ѧ�Ļ�ѧʵ����ÿ�춼�ŷ��ųɷָ��ӵ���Ⱦ�Ϊ��ijѧУ��ѧ�о�С�����������ʵ�飬�Դ��Բ��Ը�����ʵ���ʵ���ң��ף�������ȫ����������ĺ�����