��Ŀ����
���ǵ���ʳס���뻯ѧ֪ʶ�ܲ��ɷ֣���������������е���ʵ��գ�
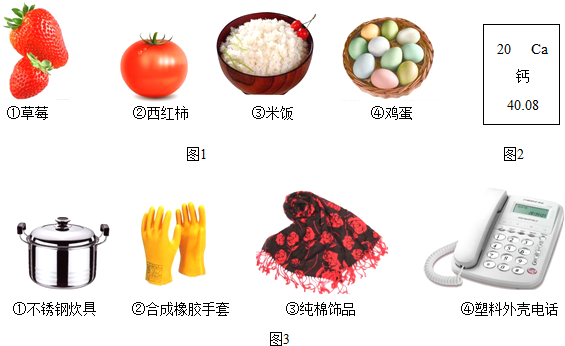
��1�����и���ʳ���е����ʺ�����ḻ����________��

��2����ͥ�����г�ʹ��������Ʒ�������õ��л��ϳɲ��ϵ���________��

��3������ȼ����[CH4?��H2O��n]��һ�ֳ�˯�ں��ߺ������Ķ�����Դ��2007��5�£����ҹ��Ϻ������ɹ����ȼ������Ʒ�������йء���ȼ������˵����ȷ����________��
�����ں��������������������������˵��ˮ���Ա����
�ۡ���ȼ����Ӧ��ǰ���������� ����ȼ��ֵ�ߡ���Ⱦ�ٵ�����Դ
��4����ͼ������ij��������վ��Ϊ�����������ľƾ��������Ĺ�森��̸̸�ƹ�ʹ�þƾ����͵�һ���ŵ�________��

�⣺��1���ٲ�ݮ��������������Ҫ����ά���أ����Դ���
��������Ҫ�������࣬���Դ���
�ܼ����к��е���ҪӪ�����ǵ����ʣ�������ȷ��
��2���л��ϳɲ�����ָ���л��߷��ӻ������ƳɵIJ��ϣ���Ҫ�����ϡ��ϳ���ά���ϳ������Ԣڢ���ȷ���ٲ�����ǽ������ϣ��ʴ��۴�������Ȼ��ά�����Ǻϳɲ��ϣ��ʴ���
��3���ٿ�ȼ���ǿ���ȼ�ϣ������ں���������Դ���
�ڸ��������غ㶨�ɣ���Ӧǰ��Ԫ�ص�����䣬ˮ������������Ԫ����ɵģ����к���̼Ԫ�أ�ˮ�����Ա���ͣ����Դ���
�ۡ���ȼ������Ҫ�ɷ��Ǽ��飬Ϊ�����Դ���Ҵ�����Ӧ��ǰ��������������ȷ��
�ܡ���ȼ������Ҫ�ɷ��Ǽ��飬��ȼ��ֵ�ߡ���Ⱦ�ٵ�����Դ��������ȷ��
��4��ʹ�þƾ����͵��ŵ���������һ���Ҵ������������Խ�Լ��ʯ��Դ��������ԴΣ����������Ⱦ�٣��������Դ���������Դٽ�ũҵ������
�ʴ�Ϊ����1���ܣ�
��2���ڢܣ�
��3���ۢܣ�
��4���ɽ�ʡʯ����Դ������������Ҳ�ɣ���
��������1�����ݲ�ݮ�������������������к��е���ҪӪ���������жϣ�
��2�������л��ϳɲ��ϵĶ���������жϣ�
��3���ٸ��ݿ�ȼ������ɽṹ�жϣ�
�ڸ��������غ㶨���жϣ�
�۸��ݡ���ȼ����Ӧ��ǰ���жϣ�
���Ǹ��ݡ���ȼ�������ŵ��жϣ�
��4������ʹ�þƾ����͵��ŵ�ش�
��������ѧ��Դ�����������Ҳ�����������������������������صĻ�ѧ֪ʶ���غ����ǵ����桢���������ķ�չ�����п��ȵ�֮һ��
��������Ҫ�������࣬���Դ���
�ܼ����к��е���ҪӪ�����ǵ����ʣ�������ȷ��
��2���л��ϳɲ�����ָ���л��߷��ӻ������ƳɵIJ��ϣ���Ҫ�����ϡ��ϳ���ά���ϳ������Ԣڢ���ȷ���ٲ�����ǽ������ϣ��ʴ��۴�������Ȼ��ά�����Ǻϳɲ��ϣ��ʴ���
��3���ٿ�ȼ���ǿ���ȼ�ϣ������ں���������Դ���
�ڸ��������غ㶨�ɣ���Ӧǰ��Ԫ�ص�����䣬ˮ������������Ԫ����ɵģ����к���̼Ԫ�أ�ˮ�����Ա���ͣ����Դ���
�ۡ���ȼ������Ҫ�ɷ��Ǽ��飬Ϊ�����Դ���Ҵ�����Ӧ��ǰ��������������ȷ��
�ܡ���ȼ������Ҫ�ɷ��Ǽ��飬��ȼ��ֵ�ߡ���Ⱦ�ٵ�����Դ��������ȷ��
��4��ʹ�þƾ����͵��ŵ���������һ���Ҵ������������Խ�Լ��ʯ��Դ��������ԴΣ����������Ⱦ�٣��������Դ���������Դٽ�ũҵ������
�ʴ�Ϊ����1���ܣ�
��2���ڢܣ�
��3���ۢܣ�
��4���ɽ�ʡʯ����Դ������������Ҳ�ɣ���
��������1�����ݲ�ݮ�������������������к��е���ҪӪ���������жϣ�
��2�������л��ϳɲ��ϵĶ���������жϣ�
��3���ٸ��ݿ�ȼ������ɽṹ�жϣ�
�ڸ��������غ㶨���жϣ�
�۸��ݡ���ȼ����Ӧ��ǰ���жϣ�
���Ǹ��ݡ���ȼ�������ŵ��жϣ�
��4������ʹ�þƾ����͵��ŵ�ش�
��������ѧ��Դ�����������Ҳ�����������������������������صĻ�ѧ֪ʶ���غ����ǵ����桢���������ķ�չ�����п��ȵ�֮һ��

��ϰ��ϵ�д�
�����Ŀ




 24�����ǵ���ʳס���뻯ѧ֪ʶ�ܲ��ɷ֣���������������е���ʵ��գ�
24�����ǵ���ʳס���뻯ѧ֪ʶ�ܲ��ɷ֣���������������е���ʵ��գ�