��Ŀ����
����Ŀ��ѧУ�о���ѧϰС��Ϊ�˲ⶨ���ؿ�ɽʯ��ʯ��̼��Ƶ�����������ȡ����һЩ��ʯ��Ʒ����ȡϡ����200 g��ƽ���ֳ�4�ݽ���ʵ�飬������£�
ʵ�� | һ | �� | �� | �� |
������Ʒ������/g | 5 | 10 | 15 | 20 |
���ɵ�CO2����/g | 1.76 | 3.52 | 4.4 | m |
��1���ϱ���m����ֵ�� ��
��2����Ӧ�п�ʯ��ʣ���ǵ� ��ʵ�飻
��3������ʯ��ʯ����̼��Ƶ����������� ��
��4��ϡ���������ʵ����������� ��
���𰸡���1��4.4����2�������ģ�
��3��80%����4��14.6%��
����������1����ʯ��ʯ����Ϊ15gʱ������50gϡ�����������ʯ��ʯû��ȫ��Ӧ�����ԣ�ʯ��ʯ���ӵ�20gʱ��50gϡ������Ȼ�����㣬���Դ�ʱ����������̼��������ȻΪ4.4g��
��2��������ʵ��50gϡ����������ʯ��ʯû��ȫ��Ӧ�����Ĵ�ʵ��ʯ��ʯ���ӵ�20gʱ��50gϡ������Ȼ�������ʯ��ʯû����ȫ��Ӧ���ʴ������ģ�
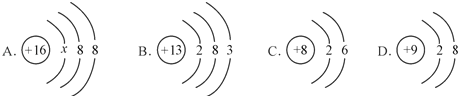
��3����10gʯ��ʯ��̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 3.52g
100��44=x��3.52g ��֮�� x=8g
ʯ��ʯ����̼��Ƶ���������=![]() =80%
=80%
��4��������4.4g������̼�������������Ϊy
CaCO3+2HCl=CaCl2+H2O+CO2��
73 44
y 4.4g
73��44=y��4.4g ��֮��y=7.3g
ϡ���������ʵ���������=![]() ��100%=14.6%
��100%=14.6%