��Ŀ����
ij��ȤС��ӹ�ͭ���Ϲ���һЩ��ͭ�̡���м��Ϊ�˲ⶨ��Щ��м��Cu2��OH��2CO3�ĺ�������С���ȡ2.22g��м�����ձ��У�Ȼ������ϡ���ᣬ�ȣ��������з�Ӧ��Cu2��OH��2CO3+2H2SO4=2CuSO4+CO2��+3H2O��ǡ����ȫ��Ӧʱ����ȥϡ����31.11g���õ������͵�����ͭ��Һ32g��������м�е��������ʼȲ�����ˮҲ�����й����ʷ�Ӧ���Լ��㣺��1����ʽ̼��ͭ[Cu2��OH��2CO3]����Է���������
��2����м�м�ʽ̼��ͭ������������
��3�����õ�������ͭ��Һ�����ʵ�����������
���𰸡���������1��������Է��������Ķ��忼�ǣ���2�������ʽ̼��ͭ�������ٸ��ݷ�Ӧ��������Һ�ļ��㷽���г���ʽ���м��㣻��3�����ݵڣ�2����������ļ�ʽ̼��ͭ�������������ͭ���������ٳ���32g���ɣ�
����⣺��1��ͭԪ�ص����ԭ��������64����Ԫ��Ϊ1����Ԫ��Ϊ16��̼Ԫ��Ϊ12��
��ʽ̼��ͭ����Է�������=64×2+��16+1��×2+12+16×3=222
��2������м��Cu2��OH��2CO3������Ϊx��
Cu2��OH��2CO3+2H2SO4=2CuSO4 +CO2��+3H2O
222 320 44
x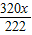
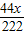
������ã�x+31.11g-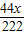 =32g
=32g
��ã�x=1.11g
��м�м�ʽ̼��ͭ����������= ×100%=50%
×100%=50%
��3����Ӧ���ɵ�CuSO4������=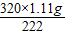 =1.6g
=1.6g
���õ�������ͭ��Һ�����ʵ���������=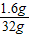 ×100%=5%
×100%=5%
�𣺼�ʽ̼��ͭ����Է�������Ϊ222����м�м�ʽ̼��ͭ����������Ϊ50%�����õ�������ͭ��Һ�����ʵ���������Ϊ5%��
�ʴ�Ϊ����1��222����2��50%����3��5%��
�������ڼ��㷴Ӧ����Һ�����е�ʽʱǧ�������ʵ������������ڣ���Ҫ�������ܳ�ȥ�Ķ�����̼��������
����⣺��1��ͭԪ�ص����ԭ��������64����Ԫ��Ϊ1����Ԫ��Ϊ16��̼Ԫ��Ϊ12��
��ʽ̼��ͭ����Է�������=64×2+��16+1��×2+12+16×3=222
��2������м��Cu2��OH��2CO3������Ϊx��
Cu2��OH��2CO3+2H2SO4=2CuSO4 +CO2��+3H2O
222 320 44
x
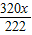
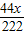
������ã�x+31.11g-
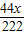 =32g
=32g��ã�x=1.11g
��м�м�ʽ̼��ͭ����������=
 ×100%=50%
×100%=50%��3����Ӧ���ɵ�CuSO4������=
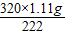 =1.6g
=1.6g���õ�������ͭ��Һ�����ʵ���������=
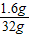 ×100%=5%
×100%=5%�𣺼�ʽ̼��ͭ����Է�������Ϊ222����м�м�ʽ̼��ͭ����������Ϊ50%�����õ�������ͭ��Һ�����ʵ���������Ϊ5%��
�ʴ�Ϊ����1��222����2��50%����3��5%��
�������ڼ��㷴Ӧ����Һ�����е�ʽʱǧ�������ʵ������������ڣ���Ҫ�������ܳ�ȥ�Ķ�����̼��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ