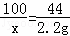��Ŀ����
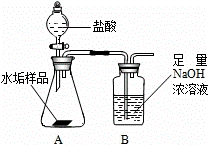
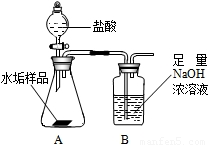
����ʹ�õ�ˮ���ײ�����һ��ˮ��������Ҫ�ɷ���̼��ƺ� ������þ��Ϊ��ȷ�ⶨˮ����������þ�ĺ�����ʵ��С��ֱ�ȡ����ͬ����ˮ����Ʒ��7.00g��������ͼ��ʾװ��������������ʵ�飬����ÿ��ʵ����װ��B�������仯��¼���±�
������þ��Ϊ��ȷ�ⶨˮ����������þ�ĺ�����ʵ��С��ֱ�ȡ����ͬ����ˮ����Ʒ��7.00g��������ͼ��ʾװ��������������ʵ�飬����ÿ��ʵ����װ��B�������仯��¼���±�
| ��һ�� | �ڶ��� | ������ | ƽ��ֵ | |
| Bװ�����ӵ����� | 2.17 | 2.22 | 2.21 | ��2.20�� |
��������ʵ�������ݺ�ش�
��1����һ��ʵ�������ݽϵ͵�ԭ��������
��2��ƽ��ÿ��ˮ����Ʒ��̼��Ƶ�����Ϊ�����ˣ�
| ��1�����ݵ�һ��ʵ�����ɵĶ�����̼û��ȫ������Bװ������ȥ���н�� ��2�����ݶ�����̼���������ÿ��ˮ����Ʒ��̼��Ƶ��������ɣ� | |
| ��� | �⣺ƽ��ֵ= ��1����һ��ʵ�����ɵĶ�����̼û��ȫ������Bװ������ȥ�����Ե�һ��ʵ�������ݽϵͣ������һ��ʵ�����ɵĶ�����̼û��ȫ������Bװ������ȥ�� ��2����ƽ��ÿ��ˮ����Ʒ��̼��Ƶ�����Ϊx CaCO3+2HCl═CaCl2+CO2��+H2O 100 44 x 2.20g
x=5.00g ��ƽ��ÿ��ˮ����Ʒ��̼��Ƶ�����Ϊ5.0g�����5.00�� |

����ʹ�õ�ˮ���ײ�����һ��ˮ��������Ҫ�ɷ���̼��ƺ�������þ��Ϊ��ȷ�ⶨˮ����������þ�ĺ�����ʵ��С��ֱ�ȡ����ͬ����ˮ����Ʒ��7.00g��������ͼ��ʾװ��������������ʵ�飬����ÿ��ʵ����װ��B�������仯��¼���±� 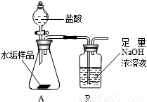
| ��һ�� | �ڶ��� | ������ | ƽ��ֵ |
Bװ�����ӵ����� | 2.17 | 2.22 | 2.21 |
|
��������ʵ�������ݺ�ش�
��1����һ��ʵ�������ݽϵ͵�ԭ����___________��
��2��ƽ��ÿ��ˮ����Ʒ��̼��Ƶ�����Ϊ___________�ˣ�
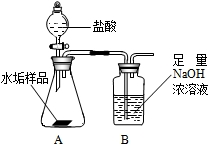
����ʹ�õ�ˮ���ײ�����һ��ˮ��������Ҫ�ɷ���̼��ƺ�������þ��Ϊ��ȷ�ⶨˮ����������þ�ĺ�����ʵ��С��ֱ�ȡ����ͬ����ˮ����Ʒ��7.00g��������ͼ��ʾװ��������������ʵ�飬����ÿ��ʵ����װ��B�������仯��¼���±�
| ��һ�� | �ڶ��� | ������ | ƽ��ֵ | |
| Bװ�����ӵ����� | 2.17 | 2.22 | 2.21 | ______ |
��1����һ��ʵ�������ݽϵ͵�ԭ����______��
��2��ƽ��ÿ��ˮ����Ʒ��̼��Ƶ�����Ϊ______�ˣ�
| ��һ�� | �ڶ��� | ������ | ƽ��ֵ | |
| Bװ�����ӵ����� | 2.17 | 2.22 | 2.21 | |
��1����һ��ʵ�������ݽϵ͵�ԭ���� ��
��2��ƽ��ÿ��ˮ����Ʒ��̼��Ƶ�����Ϊ �ˣ�
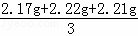 =2.20g�����2.20��
=2.20g�����2.20��