��Ŀ����
����Ŀ���Ͻ��������г����Ľ������ϡ�
(1)�����е�����Ʒ����������Ͻ�(��ͼ)��

���봿����ȣ����Ͻ��Ӳ�ȸ�____(ѡ���С��)��
���ڿ����У�����Ʒ�������γ�һ�����ܵ�����Ĥ���������á�д���γ�����Ĥ�Ļ�ѧ����ʽ��______________��
(2)�±���һЩ�������۵����ݡ�
���� | �� | Ǧ | �� | �� |
�۵�/�� | 232 | 327 | 271 | 321 |
�ٺ�����Ǧ�����Ͻ���ͼ�Ǻ����Ͻ���ij�ֽ��������������뺸���Ͻ���۵��ϵͼ�����к������ʾ����______���������������Ͻ��۵����ʱ���Ͻ���Ǧ������������Ϊ_________��
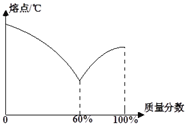
�ڱ���˿���顢Ǧ����������ɣ����۵�ԼΪ��________����
A��15��30�� B��60��80�� C��235��250�� D��300��320��
���𰸡� �� 4Al+3O2=2Al2O3 ������������ 2��3 B
��������(1)�ٺϽ��Ӳ�ȴ���������ĸ��ɷֽ�����Ӳ�ȣ������ڿ������������������÷�Ӧ�Ļ�ѧ����ʽΪ��4Al+3O2=2Al2O3��(2)�ٺ�������������Ϊ��ʱ�۵�Ҫ����������Ϊ1ʱ�۵�ߣ���ΪǦ���۵�������۵�ߣ����Ժ������ʾ���������������Ͻ��۵����ʱ��ռ60%������Ǧռ40%���Ͻ���Ǧ������������Ϊ40%��60%=2��3���ںϽ���۵��������ɳɷ��۵�Ҫ�ͣ��顢Ǧ�������������������۵���͵���231.9������Ҫѡ���۵��231.9�ͣ��Ҳ��ܺܵͣ�����Ҫ�������£�����ѡ��B��

 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д� �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�����Ŀ��ijУͬѧ��ѧ�ꡰ̼�ļ��ֵ��ʡ�һ�ں���������������ˮ��(��ͼ��ʾ)�����ֱ����������������±���ʾ�����ʣ����������Ա�ʵ�顣
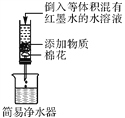
����ȡһ�����ձ���װ��ˮ���������еμӼ��κ�īˮ��������Ⱥ���ֳɵ���������ݣ��ٷֱ���������ˮ���У�ͬѧ�ǰѹ۲쵽����Һ����ɫ�仯�����¼���£�
ʵ����� | �������ʼ����� | ʵ������ |
ʵ��һ | 1 g����̿ | ��Һ�ĺ�ɫ��dz |
ʵ��� | 2 g����̿ | ��Һ�ĺ�ɫ��ʧ |
ʵ���� | 2 gľ̿ | ��Һ�ĺ�ɫ��dz |
(1)ͨ�����ϱ��ıȽϣ��㷢�ֵ������ǣ�
��______________________________________________________________
��______________________________________________________________
(2)ͨ��ʵ�飬��ó��Ľ�����______________________________(дһ�㼴��)��
����Ŀ�����м������ֲ�ͬ���ʵķ�������ȷ����( )
��� | ����������� | ���� |
A | CO��H2 | ȼ�ŵ�ľ��,�۲������ɫ |
B | ������O2 | ȼ�ŵ�ľ�� |
C | ʳ��ˮ�Ϳ�Ȫˮ | ����ζ |
D | CO��CO2 |