��Ŀ����
����Ŀ��ú��������úΪԭ�ϣ�������ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ���̡���ش��������⣺
��1������˵����ȷ����____��
A ú�Ǹ��ӵĻ�����Ҫ��̼Ԫ��
B ������Һ��ʯ����������ú�����������ȵõ��IJ�Ʒ
C úȼ���ŷŵĶ����������������γ��������Ҫ����
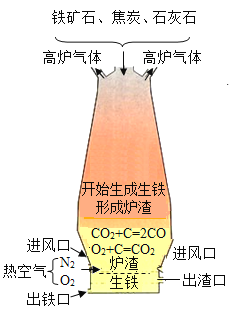
��2��ú�ļӹ���Ʒ�кܶ���;�����н�̿�㷺Ӧ������������ͼ�Ǹ�¯������ʾ��ͼ������ͼ���ش�
�ٽ�̿�����������е���Ҫ������____��
���û�ѧ����ʽ��ʾ�ó�������Ҫ�ɷ�����������������ԭ��_____��
��3�����ʳ������ϳɰ���ԭ����ú��������ˮ��������Ҫ�������£�
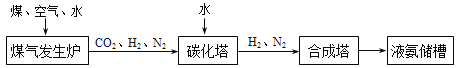
ú������¯����Ҫ��Ӧ���� C + O2 ![]() CO2���� C + H2O
CO2���� C + H2O![]() CO + H2��������Ӧ�����
CO + H2��������Ӧ�����
�е��ز��ܱ���һ����¯�¡��жϣ���Ӧ��____�������ų���������������������
���ϳɵİ�������ͨ��̼�������ȳ�ȥ�˶�����̼�ֵõ��˻���NH4HCO3����д���÷�Ӧ�Ļ�ѧ����ʽ___��
���𰸡�AC �ṩ������������ԭ��CO 3CO+ Fe2O3 ![]() 2Fe +3CO2 ���� NH3+H2O+CO2=NH4HCO3
2Fe +3CO2 ���� NH3+H2O+CO2=NH4HCO3
��������
��1��A��ú�Ǹ��ӵĻ�����Ҫ��̼Ԫ�أ�A��ȷ��
B��������Һ��ʯ��������ͨ��ʯ�ͷ���õ��IJ�Ʒ������ȷ��
C��úȼ���ŷŵĶ�����������������ˮ��Ӧ�����ᣬ��ˣ��������������γ��������Ҫ���ʣ���ȷ����ѡAC��
��2���������ڸ��������°������������е�����ԭ��������̿�����������е���Ҫ�������ṩ������������ԭ��CO����������Ҫ�ɷ����������������ķ�Ӧ����ʽΪ3CO+ Fe2O3 ![]() 2Fe +3CO2��
2Fe +3CO2��
��3���� C + O2 ![]() CO2��Ӧ�ų��������ȣ�������Ӧ������е��ز��ܱ���һ����¯�£�˵����Ӧ�� C + H2O
CO2��Ӧ�ų��������ȣ�������Ӧ������е��ز��ܱ���һ����¯�£�˵����Ӧ�� C + H2O![]() CO + H2��Ӧʱ�������������ϳɵİ�������ͨ��̼�������ȳ�ȥ�˶�����̼�ֵõ��˻���NH4HCO3�����Ը÷�Ӧ�Ļ�ѧ����ʽNH3+H2O+CO2=NH4HCO3��
CO + H2��Ӧʱ�������������ϳɵİ�������ͨ��̼�������ȳ�ȥ�˶�����̼�ֵõ��˻���NH4HCO3�����Ը÷�Ӧ�Ļ�ѧ����ʽNH3+H2O+CO2=NH4HCO3��

 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�����Ŀ��Ϊ�˲ⶨʵ�������������Ʒ�Ĵ��ȣ�ijѧϰС��ȡ2.5g����Ʒ��0.5g�������̻�ϣ����ȸû����t1ʱ��������ʲ��μӷ�Ӧ������ȴ������ʣ�������������ظ����ϲ��������γƵü���t1��t2�� t3��t4ʱ���ʣ��������������¼�������±�:
����ʱ�� | t1 | t2 | t3 | t4 |
ʣ���������/g | 2.48 | 2.34 | 2.04 | 2.04 |
����ϸ����ʵ�����ݣ��ش���������
��1����ȫ��Ӧ���������___________g
��2������Ʒ������ص���������_________��
����Ŀ��������һ�ֱ������Ȼ��Դ��
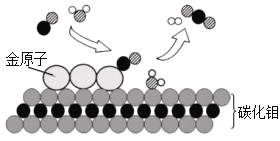
��1����ͼ�ǿ�����һЩ�������;��

��������������;����________����ͼ����ĸ��ţ���ͬ�������ڵ�����;����________��˵���������е�������_________________��
��2���������ʷе㲻ͬ����ʵ�ֻ����ķ��룬�����±������жϡ�
���� | ���� | ���� | ���� |
�е�/�� | -252.8 | -195.8 | -183.0 |
��ҵ����ȡ�����������¶���_________________��Χʱ�����Խ�Һ̬�����еĵ������������뿪��
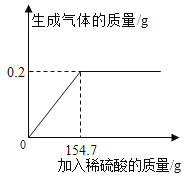
����Ŀ��Ϊ�˽�Լ��ľ��Դ��������������ʯͷֽ��ʯͷֽ�����غ�ˮ����ֳ�в����Ĵ������������Ƶá� ij��ѧ��ȤС��ͬѧΪ�˲ⶨij�ֱ�����̼��Ƶ�������������100gϡ�������μ���ʢ��12g���ǵ��� ���У���ַ�Ӧ�����ձ������ʵ����������ʾ(�������������ʲ������ᷴӦ�Ҳ�����ˮ)������� �㣺
��1�� | ��2�� | ��3�� | ��4�� | ��5�� | |
����ϡ���������/g | 20 | 20 | 20 | 20 | 20 |
�ձ������ʵ�����/g | 30.9 | 49.8 | m | 87.6 | 107.6 |
(1)������̼��Ƶ���������______(��ȷ��0.1%)��
(2)ϡ�����뱴��ǡ����ȫ��Ӧ��������Һ�����ʵ���������_____(��ȷ��0.1%)��