��Ŀ����
����Ŀ������ʢ��Ļ���ȡ��ȼ��һ���DZ��飨��ѧʽΪC3H8������ǿ��ʹ����¿��Ա���ȼ�գ�ǿ����չ�����¾���ʶ������㡣�Իش��������⣺
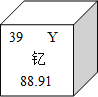
��1�����������Ҫ�����ᱡ��Ʒ�ʵ����Ͻ���ϡ���ҵ����ȡ�������ķ����ǣ�ͨ��ֽ���������Al2O3���õ���������ͬʱ��������������Ӧ�Ļ�ѧ����ʽΪ___________��������Ӧ�Ļ���������________��
��2��������������Ͻ����������ڻ�����ղػ�档������ʴ��ԭ�������ڿ��������������γ�һ��______��Ĥ����������Ʒ����������ʴ�����㻹˵���������Ͻ���ϵ������ŵ㣨����һ�㣩___________________��
��3����ȼ��桰ʥ�𡱻������ð��澵�������⡣����Ϊ���ֵ�ȼ���ṩ��_________�����ȼ��ʱ��_______��ת��Ϊ���ܺ��ܡ�
��4��������ȼ����������Ӧ�Ļ�ѧ����ʽΪ__________������ȼ�Ϸ��ϡ���ɫ���ˡ���Ҫ��ԭ����____________��
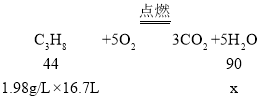
��5���人���˻��桰��ƽ�ٹ⡱�ڹ涨ʱ����ȼ��16.7L���飨�ڱ�״���£�������ܶ�Ϊ1.98g/L��������ȫȼ�տ�����ˮ�������Ƕ���____��
���𰸡�2Al2O3![]() 3Al+3O2�� �ֽⷴӦ ��������Al2O3�� �ܶ�С��㡢Ӳ�Ƚϴ�� �㹻������ ��ѧ C3H8��5O2
3Al+3O2�� �ֽⷴӦ ��������Al2O3�� �ܶ�С��㡢Ӳ�Ƚϴ�� �㹻������ ��ѧ C3H8��5O2![]() 3CO2��5H2O ����ˮ�Ͷ�����̼������Ⱦ���� 54g
3CO2��5H2O ����ˮ�Ͷ�����̼������Ⱦ���� 54g
��������
��1��ͨ��ֽ���������Al2O3���õ���������ͬʱ��������������Ӧ�Ļ�ѧ����ʽΪ2Al2O3![]() 3Al+3O2�����˷�Ӧ���ڷֽⷴӦ��
3Al+3O2�����˷�Ӧ���ڷֽⷴӦ��
��2�����ܻ��ã������������������Ӧ���ڱ�������һ�����ܵ���������Ĥ����ֹ�˽�һ���������������к�ǿ�Ŀ���ʴ�ԣ����Ͻ���ϵ��ŵ��У��ܶ�С��㡢Ӳ�Ƚϴ�ȣ�
��3������ȼ�ձ���߱�����������һ�ǵõ��Ż�㣬���Ǻ������Ӵ�������ȱһ���ɣ�����������Ϊ���ṩ�������Ա�õ��Ż�㣻ȼ���ǻ�ѧ��ת��Ϊ���ܺ����ܣ�
��4��������һ���л���ȼ�����ɶ�����̼��ˮ������ʽΪC3H8��5O2![]() 3CO2��5H2O������ȼ�����ɶ�����̼��ˮ�����������Ⱦ��
3CO2��5H2O������ȼ�����ɶ�����̼��ˮ�����������Ⱦ��
��5������ȫȼ�տ�����ˮ��������Ϊx��
![]()
x=54g��