��Ŀ����
����Ŀ����һ����ѧ֪ʶ��Ԥ���¹ڲ�����Ⱦ�з�������Ҫ���á�
��1���������Ԥ��������ԭ���൱�ڻ�ѧʵ���е�________������
��2������̿��������Ч��������еİ���������ȩ���к����壬�书�ܱ���ͨ����ǿ��ԭ���ǻ���̿����________�ԡ�
��3������N95���ֵ���Ҫԭ���Ǿ۱�ϩ![]() ��������________�����������������ϳ��������ϡ�
��������________�����������������ϳ��������ϡ�
������C919���Ϳͻ����ҹ��������Ƶģ���־���ҹ��ɻ����켼���ķ�Ծ����ش�
��1������CO��ԭ������ʯ���Ƶ������ʣ��䷴Ӧ�Ļ�ѧ����ʽΪ________��
��2���ɻ��ϵĽ�������һ���úϽ𣬺Ͻ��Ӳ��________������ڡ�С�ڡ����ڣ�������Ľ������ɻ��ϵ���˿��һ�㲻�����ʵģ���ԭ����������________��Ӧ�����⡣
��3�����������ã�������Ʒ��ʴ����ԭ��________���û�ѧ����ʽ��ʾ����
��4���ɻ��ij���Ϊ�˿��ṩ������ʳ���͡�ѩ�̡�ţ�̡��߲������ȡ��߲���������ͨ�������ṩ��Ӫ��������Ҫ��________��������һ���Ż𣬿��ù��Ǹ���ԭ����________��
��5���ɻ��������б�������������������Һ��������������Ԫ�صĻ��ϼ�Ϊ________��
�������Ȼ��ƣ�![]() ����������;��ʵ�����ù�ҵ����ʯ����������
����������;��ʵ�����ù�ҵ����ʯ����������![]() ��
��![]() ��
��![]() �����ʣ��Ʊ��Ȼ��Ƶ���Ҫ������ͼ��ʾ��
�����ʣ��Ʊ��Ȼ��Ƶ���Ҫ������ͼ��ʾ��

��֪��![]() ������ˮ��������Ҳ�������ᷴӦ���ش��������⣺
������ˮ��������Ҳ�������ᷴӦ���ش��������⣺
��1������12%��ϡ����730g����Ҫ36.5%��Ũ����________g��
��2�����ܹ����У�![]() ���뷴Ӧ�Ļ�ѧ����ʽΪ________��
���뷴Ӧ�Ļ�ѧ����ʽΪ________��
��3��������ijɷ���________���������к���![]() ��________��
��________��
��4����������õķ����ǣ�����Ũ����________�����ˡ�
��5�������Լ�a��Ŀ����________��
���𰸡����� ���� �ϳ� Fe2O3+3CO![]() 2Fe+3CO2 ���� ������ˮ 4Al+3O2=2Al2O3 ά���� �������� -1 240g Fe2O3+6HCl=2FeCl3+3H2O SiO2 Fe��OH��3 ���½ᾧ ȥ����������������
2Fe+3CO2 ���� ������ˮ 4Al+3O2=2Al2O3 ά���� �������� -1 240g Fe2O3+6HCl=2FeCl3+3H2O SiO2 Fe��OH��3 ���½ᾧ ȥ����������������
��������
��һ����1���������Ԥ��������ԭ���൱�ڻ�ѧʵ���еĹ��˲�����
��2������̿���������ԣ����������ж����壻
��3���۱�ϩ��һ�ֺϳ���ά�����ںϳɲ��ϣ�
��������1������CO��ԭ������ʯ���Ƶ������ʣ���һ����̼�ڸ����������������ijɷ���������Ӧ���������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+3CO![]() 2Fe+3CO2��
2Fe+3CO2��
��2���Ͻ��Ӳ�ȴ���������Ľ�����Ӳ�ȣ��ɻ��ϵ���˿��һ�㲻�����ʵģ���ԭ����������������ˮ��Ӧ�����⡣
��3�������������������Ӧ�����������һ�����ܵ���������Ĥ����ֹ�ڲ�������һ������������Ӧ�Ļ�ѧ����ʽΪ��4Al+3O2=2Al2O3��
��4���߲���������ͨ�������ṩ��Ӫ��������Ҫ��ά���أ�������һ���Ż𣬿��ù��Ǹ������������˸�����������ԭ����
��5���ڹ��������У���Ԫ����+1�ۣ�����Ԫ�صĻ��ϼ�Ϊ![]() ������ݻ������и�Ԫ���������ϼ۵Ĵ�����Ϊ0���ã���+1����2+2
������ݻ������и�Ԫ���������ϼ۵Ĵ�����Ϊ0���ã���+1����2+2![]() =0��
=0��![]() =-1��
=-1��
��������1����������ϡ��ǰ�䣬����Ҫ36.5%��Ũ��������Ϊ![]() ��730g��12%=
��730g��12%=![]() ��36.5%��
��36.5%��![]() =240g��
=240g��
��2�������������ᷴӦ�����Ȼ�����ˮ����ѧ����ʽΪ��Fe2O3+6HCl=2FeCl3+3H2O��
��3��������ֻ�ж������費���뷴Ӧ������������Ϊ�������裻����������������Ӧ�����˿����Ե��Ȼ������Ȼ��������������Ʒ�Ӧ��������������������������������������Ϊ��������������������
��4����Һ��������Ũ���γɱ�����Һ��Ȼ���½ᾧ���������������˺�õ����壻
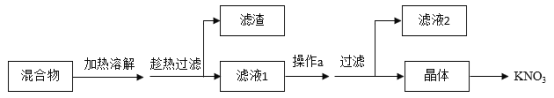
��5���������������ᷴӦ�����Ȼ��ƺ�ˮ��ǰ��IJ����м����������ƹ��������Լ����Լ�aȥ���������ơ�

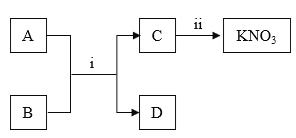
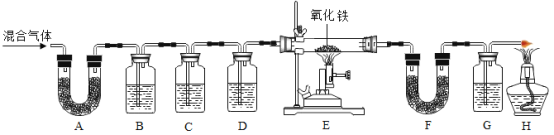
����Ŀ��ijʵ��С����̽����Ļ�ѧ����ʵ��ʱ���õ�������������Һ������������Һ��
��1����ͬѧ��С�Ľ���������Һ�����һ�𣬽������__________������˵������������Һ�����ˡ�д������ʱ������Ӧ�Ļ�ѧ����ʽ____________��
��2��ͬѧ�ǶԱ��ʵ�����������Һ�����ʵijɷֲ�������Ȥ����������̽����
��������⣩����������Һ�����ʵijɷ���ʲô��
���������룩����һ��Na2CO3
�������___________��д��ѧʽ��
���������ۣ���ͬѧȡ��������Һ����������ϡ���ᣬ�����ݲ�������Ϊ����һ��������ͬѧ��ͬ���ͬѧ�Ĺ۵㣬������_______��
�����ʵ�飩������֤����������������ʵ�鱨��
ʵ����� | ʵ������ | ʵ����� |
��ȡһ�����Ĵ���Һ���Թ��У�����___________��Һ�� �ڳ�ַ�Ӧ���ã�ȡ�ϲ�[Һ������ɫ��̪��Һ | ________ | ��������� |